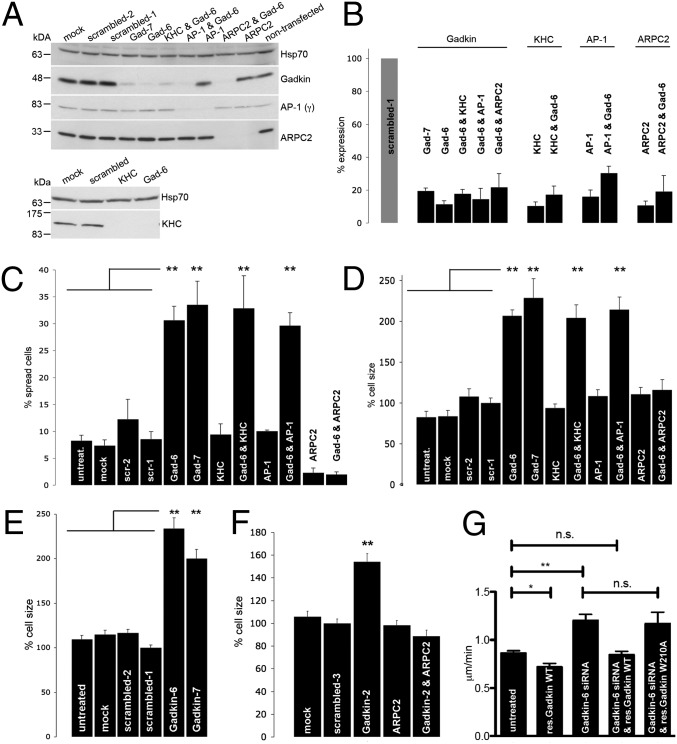
Fig. 4.
Gadkin modulates cell spreading and size via regulation of ARP2/3. (A) Representative immunoblots of lysates from cells treated with the indicated siRNAs incubated with antibodies against the proteins indicated on the right. (B) Quantification of knockdown efficiency based on immunoblotting (n = 2–4). (C and D) B16F1 cells treated with the indicated siRNAs were trypsinized and seeded onto glass coverslips. Fifteen to 20 min postplating, the fraction (%) of spread cells (C), as well as the cell size normalized to cells treated with scrambled-1 siRNA (D), was determined (n = 2–4 experiments; n = 105–312 cells per condition per experiment; **P < 0.001; one-way ANOVA plus Tukey’s post test). (E and F) B16F1 (E) respectively A431 (F) cells treated with the indicated siRNAs were fixed 24 h after plating and stained with phalloidin. Cell size was quantified and normalized to the size of cells treated with scrambled-1 respectively scrambled-3 siRNA (A431: n = 2, n = 131–266 cells per condition; B16F1: n = 3–4, n = 217–270 cells per condition; **P < 0.001; one-way ANOVA plus Tukey’s post test). (G) B16F1 cells treated with control or Gadkin-specific siRNAs transiently expressing siRNA resistant WT or ARP2/3 binding defective mutant (W210A) Gadkin-EGFP were assayed for their ability to randomly migrate. Reexpression of WT but not W210A mutant Gadkin rescued enhanced migration seen in Gadkin-depleted cells (n = 3; n = 30 cells per experiment; **P < 0.001; *P < 0.05; unpaired Student’s t test; mean ± SEM).