Abstract
Behavioural observations of reproduction and mate choice in wild fossorial rodents are extremely limited and consequently indirect methods are typically used to infer mating strategies. We use a combination of morphological, reproductive, spatial, and genetic data to investigate the reproductive strategy of a solitary endemic species, the Cape dune mole-rat Bathyergus suillus. These data provide the first account on the population dynamics of this species. Marked sexual dimorphism was apparent with males being both significantly larger and heavier than females. Of all females sampled 36% had previously reproduced and 12% were pregnant at the time of capture. Post-partum sex ratio was found to be significantly skewed in favour of females. The paternity of fifteen litters (n = 37) was calculated, with sires assigned to progeny using both categorical and full probability methods, and including a distance function. The maximum distance between progeny and a putative sire was determined as 2149 m with males moving between sub-populations. We suggest that above-ground movement should not be ignored in the consideration of mate acquisition behaviour of subterranean mammals. Estimated levels of multiple paternity were shown to be potentially as high as 26%, as determined using sibship and sire assignment methods. Such high levels of multiple paternity have not been found in other solitary mole-rat species. The data therefore suggest polyandry with no evidence as yet for polygyny.
Introduction
Mammals display a wide range and flexibility of behavioural and social interactions (e.g. [1]), which is well exemplified in their breeding systems. The most common mammalian mating system is polygyny [2], [3] where male mate acquisition ranges from opportunistic approaches [4], to the competitive attainment of a social or spatial position that confers improved access to females (e.g. [5]). In some species spatial proximity to potential mates reduces aggression and facilitates mating opportunities [6], [7]. Attenuated aggression through proximity may be particularly important for subterranean species which experience high energetic costs associated with extending burrow systems in order to locate potential mates.These energetic restrictions may be relaxed by the adoption of alternate strategies such as surface movement [8] or the use of adjoining burrow systems. Although males commonly attempt to monopolise females to improve reproductive success, they seldom successfully exclude all competing suitors [9]. Solitary multiparous mammalian species that do not form pair-bonds may thus be expected to have high levels of multiple paternity (e.g. [10]).
The inference of mating system from morphological information is well established (e.g. [11]) but can be problematic, particularly where mixed mating systems are found [12]. Male reproductive success is often highly variable [13] due in part to interplay between multiple mating strategies which vary both in frequency and temporally [14]. The apparent adoption of one mating system often belies complexities such as extra pair copulations [15]. Unravelling the mating dynamics in cryptic systems such as subterranean organisms is reliant on comprehensive investigations incorporating genetic, spatial, and morphological information (e.g. [16]). While familial relationships can be determined directly through genotypes, population level dynamics can be inferred from population characteristics such as reproductive skew and associated discrepancies in sex ratio [17].
The family Bathyergidae is a group of subterranean rodents found throughout sub-Saharan Africa [18]. Initially eclipsed by the discovery of eusociality in the social genera (e.g. [19]) solitary mole-rat species have only recently begun to receive research interest [8], [20], [21]. The Cape dune mole-rat, Bathyergus suillus is one of two solitary members of the genus Bathyergus [22] which is largely restricted to mesic regions [23] characterised by a sandy substrate, in the South African Western Cape [18]. Little is known about the mating system in B. suillus and based on morphology conflicting suggestions of both polyandry and polygny have been proposed [24], [25]. Assumptions about mating systems have been cautioned in the absence of genetic data [26]. Here we use a combination of demographic, morphological, spatial, reproductive, and genetic data on a large free-ranging population of B. suillus to shed light on the mating system of this endemic, fossorial rodent.
No demographic information is available for the Cape dune mole-rat and no population genetic information has been presented previously. Primarily we take a genetic approach to characterise the mating system in this subterranean rodent. We attempt to determine the degree to which a subterranean ecology limits the distance over which a male may sire progeny. Additionally, we use morphological and sex ratio information to give further insight into the mating dynamics of this species.
Methods
Sampling
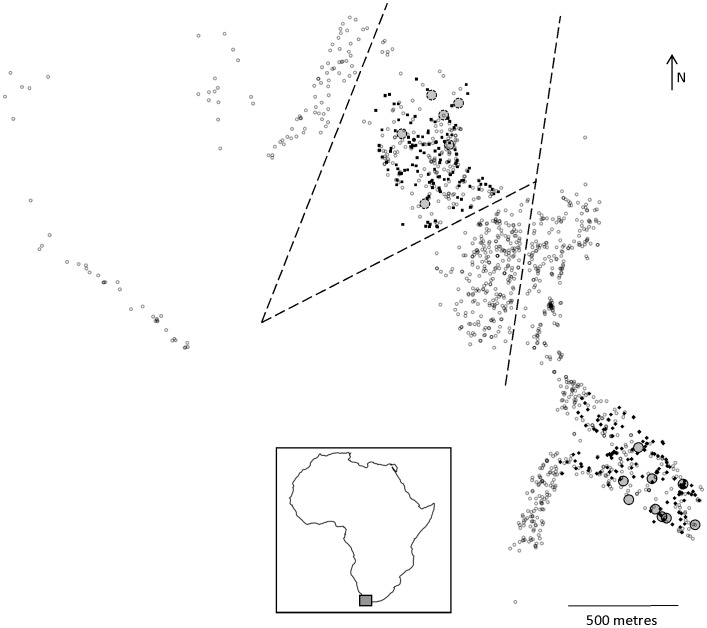
Morphological, spatial and reproductive data were obtained for 1350 B. suillus individuals that were captured and humanely euthanised as part of an eradication program run by the Airports Company of South Africa at Cape Town International Airport (see [27] for details). All procedures conformed to the guidelines of the American Society of Mammalogists [28]. The collection and processing of all biological material used in this study was approved by the University of Cape Town Animal Ethics Committee (AEC#:2003/V&/JOR). Muscle tissue samples were taken and stored in ethanol. Included in the sampling were 21 gravid females. Spatial and genetic data were analysed for a subset of the total sample from two different regions within the airport grounds that were separated by below ground barriers (runways) that prevented any subsurface movement (Fig. 1). We used these two regions to investigate the relationship between paternity and distance from sampled pregnant females. Region 1 contained nine gravid females (2-3 progeny per litter, n = 23 total) and a total of 143 potential sires (all males within 400 m of a sampled female). Region 2 contained six gravid females (1-4 progeny per litter, n = 14 total) with 152 potential sires (Fig. 1). DNA samples were collected for all females with progeny and their litters, as well as 132 males from region 1 and 139 males from region 2. An additional nine ‘transient’ individuals caught above-ground and hence with no location information were treated as potential sires for both regions.
Figure 1. Map of B. suillus individuals sampled.
The south-eastern study area is Region 1 showing study females (filled grey circles, solid lines) and potential sires (black diamonds). Region 2 is the northern study area showing study females (filled grey circles, dashed lines) and potential sires (black squares). Tarred runways and access roads representing potential barriers to subterranean movement are represented by dashed lines. Sampled individuals not genotyped are also shown (small unfilled circles).
Genotyping
Genotypes were generated from 19 microsatellite markers developed for B. suillus and related species; BS01, BS07 ([29], GenBank accession numbers; HQ186238-45); CH1, CH3, DM1, DM5, DM7 ([30], GenBank accession numbers; AF380165-75); Gcap02, Bsuil06, Bsuil04, Chott03, Cmech04, Bsuil02, Gcap07, Bsuil01, Chott05, Cmech03, Gcap10, Cmech09 [31]. These markers were applied in four multiplexes using the Qiagen Multiplex kit with standard conditions (45 cycles, 60°C annealing temperature). Heterozygosity scores and linkage disequilibrium for all locus pairs were calculated in GENETIX4.05 [32], as well as population-level summary statistics. All loci were tested for conformation to Hardy-Weinberg Equilibrium and for the presence of null alleles using GENEPOPv4.0.10 [33]. A migrant analysis was performed using GENECLASS2 [34]. Genotyping error rate across the dataset was also quantified by repetition of ∼10% of the individuals across all loci.
Capture Location and Spatial Analyses
GPS points were taken at the point of capture for all individuals sampled, and coordinates were mapped using ARCGIS 10 [35]. For the purposes of this study the capture coordinates were applied as if they were the centre of the home burrow system. We calculated mean distances between all males and females in both regions 1 and 2. We used an individual-based ‘isolation by distance’ approach across just the males, under an area model using GENEPOPv4.0.10 [33] to investigate the relationship between spatial patterns and genetic variation in both regions.
Parentage Analysis
In accordance with previous recommendations [36] two statistical approaches were applied to assign parentage based on the genetic data:
(i) Categorical allocation
The most likely sires are calculated from the non-excluded putative sires within a likelihood framework. Calculations are based on the probability of the parental genotype producing the alleles required to construct the genotype of the progeny. An advantage of this approach is that it allows the accommodation of scoring errors and mutations. Sire determinations were made using the CERVUS [37] algorithm (within the R package MASTERBAYES; [38] for paternity assignment to give the single most likely sire for each of the progeny.
(ii) Full probability
Here the specified model allows inclusion of other explanatory variables in addition to the genotype information according to the algorithm of MASTERBAYES [38]. Spatial information was included in the sire assignment to compare against the basic categorical allocation. A further advantage of this approach is the inclusion of a measure of uncertainty.So as to include the transient males into the distance analysis they were given the same geographic location outside the bounds of the region to reflect their unknown origin.
For both of these approaches analyses were performed through the MASTERBAYES [38] application in R under two scenarios: Each statistical approach was modelled for the situation assuming all sires are present (Allsire) and adopting the known rates of sire absenteeism (USsire). Absent males numbered 11 in region 1 and 13 in region 2. Sire assignment was determined separately for each region and then repeated using all sampled males from both regions. It is known that genotyping error of as low as 1% can have a strong effect on paternity assignment and exclusion [39]. We considered genotyping error rates for the dataset in the analysis employing values of 0.1, 1, and 5% for comparison. Maximum likelihood initial parameterisations are used as default with parameters being estimated by the model. The maximum likelihood pedigree was fixed with regard to the inclusion of known mothers. The two approaches employed here differ in their treatment of genotyping error; The CERVUS approach assumes the second allele in an erroneous genotype is also mis-scored, and that the true genotype is a reflection of genotype frequency in the population [40]. The MASTERBAYES error is calculated as equally likely across all alleles, with a second error term for the likelihood of the dropout of alleles from a heterozygote genotype [41].
Relatedness and Multiple Paternity
Relatedness was measured and the distribution of relatedness values described within each region using the ML-RELATE application [42]. This application calculates the maximum likelihood relationship between individuals based on genetic data. Relatedness was calculated between littermates to further investigate the incidence of multiple paternity across all 19 litters with two or more siblings. The analysis was performed according to the ‘Matrix’ setting for comparison of multiple individuals. Total allele numbers at each locus in the genotypes of those nine litters containing three or more progeny were also compiled as a third means by which to estimate multiple paternity.
Morphology and Sex
Total body length, mass, sex, and reproductive status measurements were recorded (sensu [27]) for 1350 individuals. Means for male and female length and body mass were compared using a T- test under the null hypothesis of no difference between distributions. The shape of the frequency distribution was modelled for both length and mass in each gender using the MCLUST [43] application. A sub-sample of females was investigated for uterine scarring and pregnancy (n = 518). All sex ratios were evaluated for significance using a Chi-Squared test (null hypothesis no deviation from 1∶1). Molecular sexing was used to examine sex ratios of foetuses, using the DBY markers and protocols developed for Heterocephalus glaber [44]. DBY amplification was less efficient in B. suillus than H. glaber and thermocycling conditions were altered to 45 cycles at an annealing temperature of 45°C. As absence of DBY amplification indicates a female, individual DNA was confirmed using mitochondrial DNA 16S amplification prior to a minimum of three independent DBY amplifications with positive controls.
Results
Genotyping
Deviations from Hardy-Weinberg Equilibrium and estimates of null alleles are shown in Appendix S1. Locus D1 has a highly significant heterozygote deficiency (P<0.001) and an estimated null allele frequency of 0.32, above arecommended 0.2 upper bound [45]. For these reasons locus D1 was excluded from further analyses. The test for linkage disequilibrium showed no significant association between alleles across loci (all p>0.05). Population genetic summary statistics are given in Table 1. There was a low but highly significant genetic differentiation (FST = 0.018, P<0.001) between the two regions (using ten representative members of each sex from each region). Detection of first generation migrants suggested 20 migrants each way between the two regions at the most strict assignment level (P = 0.001). Genotyping error was calculated as being 0.018%.
Table 1. Genetic summary statistics for B.suillus individuals for region 1, region 2, and total adults (including both regions and transient males).
| HE | HO | A | |
| Region 1 | 0.69 | 0.64 | 8.3 |
| Region 2 | 0.67 | 0.62 | 8.1 |
| Total | 0.69 | 0.63 | 9.1 |
Expected and observed heterozygosity (HE, HO), and number of alleles per locus (A).
Geographical Information
The mean distances within and between the sexes in regions 1 and 2 are provided in Table 2. A Mantel test of genetic distance against geographic distance indicated no statistically significant correlation within regions (10000 permutations; P = 0.001; number of pairs = 10153, 11476 for region 1 and 2 respectively), suggesting no pattern of natal philopatry in males across this site.
Table 2. Mean regional separation distances in metres between members of each gender (standard deviations in parentheses).
| Region 1 | Region 2 | |||
| Male | Female | Male | Female | |
| Male | 326(198) | 276(123) | ||
| Female | 336(199) | 343(205) | 249(134) | 277(155) |
Parentage Analysis
Sire assignment to progeny was seen to be affected by statistical approach and whether all males were considered sampled (Appendix S2). Including distance as a variable in the full probability approach did not change sire assignments. Subsequent to initial analyses and in consideration of the error rate of 0.018% determined for the dataset, assigned sires were considered only if two or fewer mismatches were present (total alleles in each assignment taking into account dam: 3*(18*2)*0.018 = 1.9 mismatches). Due to the low level of within region sire assignment according to these criteria, results presented are for the inclusion of all sampled males from both regions in each analysis. When all males were assumed sampled (ALLsire) 10/23 progeny were assigned sires by one or both methods in region 1, and 9/14 in region 2. Methods agreed on five assignments in region 1, and three in region 2. For the remaining assignments only one method assigned a sire in each case apart from two instances in region 2 where the two methods assigned different sires. When the assumption of all males sampled was relaxed (USsire) two assignments were made in region 1 (both methods in agreement; all probability>0.95) with the full probability method assigning a further three at lower confidence (probability<0.6). In region 2 a single USsire assignment was made in region 2 by the categorical approach only (probability = 0.23). Alteration of error parameter did not affect sire assignments but decreasing error increased each assignment probability by an average 0.04 in region 1 and 0.03 in region 2 in the categorical approach only.
Calculating Mate Acquisition Distances
We inferred mate acquisition distances in two ways from these data: Level of sire assignment (i.e. proportion of unsampled sires), and from direct measurements for sire-progeny assignments (Appendix S2). The drop in assignment rate when the possibility of unsampled males is introduced suggests that a potentially high proportion of unsampled males have travelled further than the sampling radius to sire offspring. Sampling radius is a minimum of 400 m for region 1 and ∼200 m for region 2. The greatest sire-progeny distance calculated for the ALLsire model where both approaches gave the same sire was 2149 m. The greatest consensus sire distance in the more conservative USsire model was 34 m. Distances above 1000 m represent movement between regions.
Determination of Multiple Paternity and Relatedness
Two of nine litters showed evidence of multiple paternity at a single locus (three or more non-maternal alleles present), a further two litters showed evidence of multiple paternity over two loci. Using the statistical approach of the MLrelate algorithm for determining sibship, five of 19 litters were found to contain half-siblings. We also inferred multiple paternity from those sire assignments where different males were assigned to a single litter with a probability of assignment of at least 0.8; Sire assignments across methods for the 15 litters suggest two litters contained two siblings attributed to the same sire, one attributed siblings to two different sires, and nine did not have more than one sire assignment for comparison. Relatedness estimates for region 1 and 2 were 0.05 and 0.06 (variance 0-0.67 and 0-0.83; number of pairs 13366 and 12561 respectively). Inter-litter relatedness was low within each region (0.025, 0.028 respectively) and both were significantly different from the regional relatedness (T-test;region 1, df = 10078, P<0.0001; region 2, df = 10516, P = 0.02).
Morphometrics and Sexing
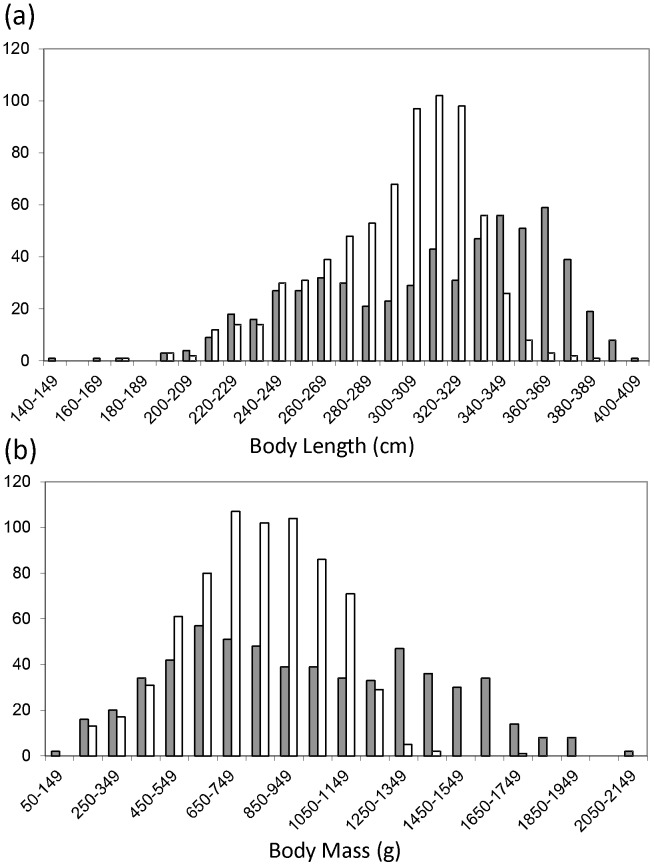
Overall mean values showed that males were significantly longer (mean = 311 mm, T-test; df = 938, P>0.0001) and heavier (mean = 955 g, T-test; df = 935, P>0.0001) than females (788 g, SD = 243; 294 mm, SD = 33, respectively) (Fig. 2a and 2b). Frequency distributions of body length and body mass reveal a unimodal distribution for females. Both length and body mass of males were best explained by models with two components (Appendix S3; length = 275, 350 mm, mass = 650,1400 g). Of the 518 females sampled 36% had uterine scarring, while 12% were pregnant on capture. The adult sex ratio for the study site was significantly female biased with a ratio of 0.84∶1 (df 1, X2 9.8, P>0.001, n = 1305) whilst the sex ratio of unborn foetuses from 21 adult females was 0.76∶1 (df 1, X2 0.96, NS, n = 51).
Figure 2. Distribution of morphological measurements by sex.
Body length (a) and body mass (b) distributions for sampled male (grey) and female (white) Cape dune mole-rats, B. suillus.
Discussion
In this study, genetic analyses have provided evidence for multiple paternity in a fossorial, solitary rodent with males appearing to travel considerable distances to acquire mates. This mirrors the findings in another solitary rodent, Heliophobius argenteocinereus [8]. These data suggest a mating system with males competing with one another for access to females and the latter accepting multiple partners.
Population Structure of B. suillus
Population level genetic data of the Cape Dune mole-rat reveal a diverse (HE = 0.69) population occurring at this site. There is no isolation by distance in either subsample of the population studied. We cannot infer philopatry as noted in other rodents (e.g. [46]) and likely given a short presumed generation time (1–2 years, based on similar species; [47]), although the lack of data from females forestalls a definite conclusion. The population at this study site is not panmictic; there is a low but significant genetic differentiation of the two physically disparate subsamples taken within the population (FST, 0.01, P>0.001). This result is largely expected for a fossorial species with low vagility [48], [49] due to the high costs associated with movement below ground [50]. However the migrant analysis does suggest that bidirectional movement occurs between the two regions subsampled in this study. B. suillus are observed moving above-ground and may thus use this energetically cheaper form of locomotion to reduce the costs of dispersing over this 1 km distance. The much smaller naked mole-rat, Heterocephalus glaber, has been documented moving over 2 km from its natal burrow when dispersing [48].
Can we Determine a Limit to Distances Across Which a Sire Will Father Progeny?
Cape dune mole-rats are strictly solitary and highly aggressive to conspecifics within laboratory settings (Bennett and Faulkes 2000). Consequently, the type and frequency of social interactions within and between the sexes remains almost completely unknown. It has been suggested that male B. suillus will position burrows adjacent to those of females [51] with longer tunnels increasing the number of females as nearest neighbours and hence improving the chances of successful mate acquisition (sensu [52]). Tunnel lengths have been shown to extend for up to 400 m with as much as 140 m of new tunnel excavated per month [24].
In this study we find a surprisingly low sire assignment rate given known burrow extent and considering the breadth of sampling of potential sires. Females in this study site are located a mean distance of 340 m from one another allowing males to position themselves between multiple female burrow systems. As such we might expect sire assignment distances to be below this inter-female distance due to optimal positioning. The maximum sire-to-progeny distance identified here is 2149 m assuming that all sires were indeed sampled. It is unlikely that a male would have burrow connections to an adjacent burrow system beyond a 500 m straight distance. How male B. suillus travel beyond adjacent burrow systems is currently unknown. For some mole-rat species, surface movement is avoided to the extent that even dispersal is known to occur under the ground [53]. Mole-rat vision is rudimentary [54] and thus surface activity would expose individuals to high risks of predation. Despite this, above-ground travel has been invoked for males siring offspring over several hundred metres away in a low density H. argenteocinereus population [8]. The combination of the low assignment rate and high upper limit to sire-progeny distances seen in this study supports this suggestion that males of subterranean species may be much less reluctant to travel above-ground than previously thought. Another clue in the data presented is the significantly lower relatedness within the progeny in each region, again implicating paternity from non-resident males. It is likely that above-ground movement is directed through a combination of auditory and olfactory cues. Seismic signals are known to be used for communication in mole-rats [55], in the form of ‘drumming’ with the hind feet. It has been noted that Georychus capensis have sexual differences in drumming behaviour [47], and urine is known to convey information regarding sexual condition in other subterranean rodents (e.g. Ctenomys talarum [56]).These sensory cues will allow identification of potential mates without entering unfamiliar burrow systems.
How Common is Multiple Paternity?
Polygyny is typical in mammalian reproduction and is strongly influenced by male behaviour such as competition, spatial organisation, and strategies refined to maximise reproductive success [57]. Despite male attempts to associate spatially with females, solitary females are difficult to monopolise [58]. Multiple paternity within litters is a common feature of polygynous systems and can reveal to some extent the interactions occurring in a cryptic system where behavioural observation is difficult. The real proportion of litters exhibiting multiple sires in the B. suillus population investigated here is likely to be somewhere close to the 5/19 predicted by within-litter sibship. Due to the potentially strong influences of genotyping error in allele counts to determine multiple paternity, and the low sire assignment rate seen here, this figure cannot be specified more accurately. Based on the probability of differential gamete inheritance we might allow for a 25% confidence interval around this estimate so potentially a proportion of 0.20-0.33 litters will show multiple paternity.This estimate is higher than the incidence of multiple paternity seen in other solitary mole-rat species (H. argenteocinereus, ∼10% [8]; Spalax ehrenbergi, ∼0% [21]).
High levels of multiple paternity (up to 80%) have been recorded for rodents with low population viscosity [59]. High levels of multiple paternity might be expected in this study population because of the artificially large size and high density of this population within a predominantly human modified habitat with limited natural predators. High density would increase mating opportunities by bringing opposite sexed individuals into closer physical proximity. Corresponding selection for strong inbreeding avoidance mechanisms might also be expected, as seen in some vole species [60].
The data here suggest that polyandry occurs in this population. Female fitness returns from increasing the number of potential fathers could be those of sperm competition [62]. It is interesting to note that multiple paternity is seen even in socially monogamous mole-rat breeding systems; the common mole-rat (Cryptomys hottentotus) is one such system with extra-pair mating seen between colonies [62]. Extra-pair extra-colonial mating has been hypothesised as a mechanism for inbreeding avoidance, characterised by increased within-litter heterozygosity [63]. Extra-pair paternity is also seen in such eusocial systems as the naked mole-rat [64], [65]. In these systems, high relatedness (coefficients of 0.5 or greater) results in a higher fitness trade-off between philopatry and assisting the survival of siblings over the risks of dispersal to form new colonies [66]. We might expect that the reduction in coefficient of relatedness associated with multiple paternity would reduce the fitness benefit of philopatry sufficiently to favour dispersal. For eusocial colonies this implies either a very high cost of dispersal, or that multiple paternity is present only at low levels. Absence of such social restrictions in the solitary species, such as B. suillus, would imply that multiple paternity is expected to occur at a much higher level.
Does Morphological Information or Sex Ratio Help Define Population Dynamics?
It has been noted that male B. suillus growth rate declines relative to that of the females at around two to three years of age [67]. High variation in male mass may reflect a period where males lose condition as they either need to search extensively for females or compete with other males for access to females. In some species mass or condition is proposed to determine dispersal distances [46]. Dispersing males are likely to experience higher mortality, successful individuals being those larger males able to establish and defend their burrows resulting in the observed bi-modal size distribution. High male mortality may explain the female biased sex ratio on this site. Sex ratios have the potential to reveal cryptic patterns within the dynamics of mating systems and among different sources of selective pressures. Sex-allocation theory stipulates an even number of each sex unless there are differential costs or fitness returns [68], [69]. Although not significant, a skewed sex ratio can be seen from birth in this population (0.76, n = 51) perhaps suggesting a predisposition of the mating system such as condition dependant sex allocation [70], but more data would be needed to confirm this.
As the first population level genetic study on B. suillus the information provided here gives an invaluable insight into this species. It is likely that the high degree of anthropogenic encroachment on organisms such as mole-rats will result in lowered connectivity to other areas. Population isolation has undoubtedly become more commonplace, particularly in low vagility subterranean organisms, and studies of populations such as this one are useful in gauging the result of this isolation. Such insights into the incidence of multiple paternity and polyandry in conjunction with above ground movement described here allow greater understanding of these subterranean organisms. Only through knowledge of these behavioural processes will we be able to describe those selective factors responsible for the generation of the enormous diversity of lifestyles noted in this unusual family of rodents.
Supporting Information
Summary information for each locus including number of alleles (A), frequency of null alleles (Null), expected and observed heterozygosity (HO, HE), and FIS (Probability of significant deviation from Hardy-Weinberg equilibrium; P<0.05 = *, P<0.01 = **, P<0.001 = ***).
(DOCX)
Sire assignments for regions 1 (a) and 2 (b) across all models showing sire identity (sire-progeny distances in metres), probability of assignment, and number of mismatches between sire and progeny genotypes (NA denotes no sire assignment made).
(DOCX)
Plots showing the shape and number of either equal (E) or variable (V) volume components; (a) male length, the best BIC values were: V,2 (-6255), V,3 (-6264), E,2 (-6270), and (b) male mass, the best BIC values were:E,2 (-8853.623), V,2(-8858), E,3 (-8866).
(TIF)
Acknowledgments
We would like to extend our gratitude to two anonymous reviewers for helpful comments.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The authors are grateful to the Department of Science and Technology and the National Research Foundation for funding from the South African Research Chairs Initiative Chair for Mammal Behavioural Ecology and Physiology awarded to NB. Funding was also provided by the University of Pretoria in provision of TB’s postdoctoral fellowship. This work is based upon research supported by the National Research Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Schradin C, Lindholm AK, Johannesen J, Schoepf I, Yuen C-H, et al. Social flexibility and social evolution in mammals: a case study of the Africa striped mouse (Rhabdomys pumilio). Mol Ecol. 2011;21(3):541–53. doi: 10.1111/j.1365-294X.2011.05256.x. [DOI] [PubMed] [Google Scholar]
- 2.Kleiman DG. Monogamy in mammals. Q Rev Biol. 1977;52:39–69. doi: 10.1086/409721. [DOI] [PubMed] [Google Scholar]
- 3.Rutberg AT. The evolution of monogamy in primates. J Theor Biol. 1983;104:93–112. doi: 10.1016/0022-5193(83)90403-4. [DOI] [PubMed] [Google Scholar]
- 4.Porschmann U, Trillmich F, Mueller B, Wolf JBW. Male reproductive success and its behavioural correlates in a polygnous mammal, the Galapagos sea lion (Zalophus wollebaeki). Mol. 2010;Ecol19:2574–2586. doi: 10.1111/j.1365-294X.2010.04665.x. [DOI] [PubMed] [Google Scholar]
- 5.Simone C. Marco A. Do antlers honestly advertise the phenotypic quality of fallow buck (Damadama) in a lekking population? Ethology. 2011;117:133–144. [Google Scholar]
- 6.Ostfeld RS. The ecology of territoriality in small mammals. Trends Ecol Evol. 1990;5:411–415. doi: 10.1016/0169-5347(90)90026-A. [DOI] [PubMed] [Google Scholar]
- 7.Randall JA, Hekkala ER, Cooper LD, Barfield J. Familiarity and flexible mating strategies of a solitary rodent, Dipodomys ingens. Anim Behav. 2002;64:11–21. [Google Scholar]
- 8.Patzenhauerova H, Bryja J, Sumbera R. Kinship structure and mating system in a solitary subterranean rodent, the silvery mole-rat. Behav Ecol Sociobiol. 2010;64:757–767. [Google Scholar]
- 9.Stacey PB. Female promiscuity and male reproductive success in social birds and mammals. Am. 1982;Nat120:51–64. [Google Scholar]
- 10.Roy Nielsen CL, Nielsen CK. Multiple paternity and relatedness in southern Illinois raccoons (Procyon lotor). J Mammal. 2007;88:441–447. [Google Scholar]
- 11.Coker CR, McKinney F, Hays H, Briggs SV, Cheng KM. Intromittent organ morphology and testis size in relation to mating system in waterfowl. The Auk. 2002;119:403–413. [Google Scholar]
- 12.Leutenegger W, Lubach G. Sexual dimorphism, mating system, and effect of phylogeny in De Brazza’s monkey (Cercopithecus neglectus). Am J Primatol. 1987;13:171–179. doi: 10.1002/ajp.1350130207. [DOI] [PubMed] [Google Scholar]
- 13.Trivers RL, Willard DE. Natural selection of parental ability to vary the sex ratio of offspring. Science. 1973;179:90–92. doi: 10.1126/science.179.4068.90. [DOI] [PubMed] [Google Scholar]
- 14.Schradin C, Lindholm AK. Relative fitness of alternative reproductive tactics in a mammal varies between years. J Anim Ecol. 2011;80:908–917. doi: 10.1111/j.1365-2656.2011.01831.x. [DOI] [PubMed] [Google Scholar]
- 15.Goossens B, Graziani L, Waits L, Farand E, Magnolon S, et al. Extra-pair paternity in the monogamous Alpine marmot revealed by nuclear DNA microsatellite analysis. Behav Ecol Sociobiol. 1998;43:281–288. [Google Scholar]
- 16.Waser PM, Hadfield JD. How much can parentage analyses tell us about precapture dispersal? Mol Ecol. 2011;20:1277–1288. doi: 10.1111/j.1365-294X.2011.05002.x. [DOI] [PubMed] [Google Scholar]
- 17.Rosenfeld CS, Roberts RM. Maternal diet and other factors affecting offspring sex ratio: A review. Biol Reprod. 2004;71:1063–1070. doi: 10.1095/biolreprod.104.030890. [DOI] [PubMed] [Google Scholar]
- 18.Skinner JD, Chimimba CT. The mammals of the southern African subregion. Cambridge University Press, Cape Town. 2005.
- 19.Jarvis JUM. Eusociality in a mammal: cooperative breeding in naked mole-rat colonies. Science. 1981;212:571–573. doi: 10.1126/science.7209555. [DOI] [PubMed] [Google Scholar]
- 20.Herbst M, Jarvis JUM, Bennett NC. A field assessment of reproductive seasonality in the threatened wild Namaqua dune mole-rat (Bathyergus janetta). J Zool. 2004;263:259–268. [Google Scholar]
- 21.Malik A, Frenkel Z, Hernandez A, Band M, Nevo E, et al. Characterisation of paternity relationships in the mole rat Spalax ehrenbergi by microsatellite genotyping. Pop Ecol. 2011;53:501–510. [Google Scholar]
- 22.Bennett NC, Faulkes CG. African Mole-rats: ecology and eusociality. Cambridge University Press, Cambridge. 2000.
- 23.Jarvis JUM, O’Riain MJ, Bennett NC, Sherman PW. Mammalian eusociality: a family affair. Trends Ecol Evol. 1994;9:47–51. doi: 10.1016/0169-5347(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 24.Davies KC, Jarvis JUM. The burrow systems and burrowing dynamics of the mole-rats Bathyergussuillus and Cryptomyshottentotus in the fynbos of the south-western Cape, South Africa. J Zool. 1986;209:125–147. [Google Scholar]
- 25.Kinahan AA, Bennett NC, O’Riain MJO, Hart L, Bateman PW. Size matters: genital allometry in an African mole-rat (Family: Bathyergidae). Evol Ecol. 2007;21:201–213. [Google Scholar]
- 26.McEachern MB, McElreath RL, Van Vuren DH, Eadie JM. Another genetically promiscuous ‘polygynous’ mammal: mating system variation in Neotoma fuscipes. Anim Behav. 2008;77:449–455. [Google Scholar]
- 27.Hart L, O’Riain MJ, Jarvis JUM, Bennett NC. Is the Cape dune mole-rat, Bathyergus suillus a seasonal or aseasonal breeder? J Mammal. 2006;87:1078–1085. [Google Scholar]
- 28.ANIMAL CARE AND USE COMMITTEE. Guidelines for the capture, handling, and care of mammals as approved by the American Society of Mammalogists. J Mammal. 1998;79:1416–1431. [Google Scholar]
- 29.Bray TC, Bloomer P, Bennett NC. Low levels of polymorphism at novel microsatellite loci developed for bathyergid mole-rats from South Africa. Conserv Genet Resour. 2011;3:221–224. [Google Scholar]
- 30.Burland TM, Bishop J, O’Rian MJ, Faulkes CG. Microsatellite primers for the African mole-rat genus Cryptomys and cross-species amplification within the family Bathyergidae. Mol Ecol Notes. 2001;1:311–314. [Google Scholar]
- 31.Ingram CM. Unpublished thesis; The evolution of nuclear microsatellite DNA markers and their flanking regions using reciprocal comparisons within the African mole-rats (Rodentia: Bathyergidae). Texas A&M University. 2005.
- 32.Belkhir K, Borsa P, Chikhi L, Raufaste N, Bonhomme F. GENETIX 4.05, Logiciel Sous WindowsTM Pour la Genetique Des Populations. Laboratoire Genome Populations, Interactions. CNRS UMR 5000. Universite de Montpellier II, Montpellier, France. 2004.
- 33.Rousset F. GENEPOP’007: a complete re-implementation of the GENEPOP software for Windows and Linux. Mol Ecol Resour. 2008;8:103–106. doi: 10.1111/j.1471-8286.2007.01931.x. [DOI] [PubMed] [Google Scholar]
- 34.Piry S, Alapetite A, Cornuet J-M, Paetkau D, Badouin L, et al. GENECLASS2: A software for genetic assignment and first-generation migrant detection. J Hered. 2004;95:536–539. doi: 10.1093/jhered/esh074. [DOI] [PubMed] [Google Scholar]
- 35.ESRI. ArcGIS Desktop: Release 10. Redlands, CA: Environmental Systems Research Institute. 2011.
- 36.Jones AG, Small CM, Paczolt KA, Ratterman NL. A practical guide to methods of parentage. Mol Ecol Resour. 2009;10:6–30. doi: 10.1111/j.1755-0998.2009.02778.x. [DOI] [PubMed] [Google Scholar]
- 37.Kalinowski ST, Taper ML, Marshall TC. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol Ecol. 2007;16:1099–1106. doi: 10.1111/j.1365-294X.2007.03089.x. [DOI] [PubMed] [Google Scholar]
- 38.Hadfield JD, Richardson DS, Burke T. Towards unbiased parentage assignment: combining genetic, behavioural and spatial data in a Bayesian framework. Mol Ecol. 2006;15:3715–3730. doi: 10.1111/j.1365-294X.2006.03050.x. [DOI] [PubMed] [Google Scholar]
- 39.Hoffman JI, Amos W. Microsatellite genotyping errors: detection approaches, common sources and consequences for paternal exclusion. Mol Ecol. 2005;14:599–612. doi: 10.1111/j.1365-294X.2004.02419.x. [DOI] [PubMed] [Google Scholar]
- 40.Marshall TC, Slate J, Kruuk LEB Pemberton JM. Statistical confidence for likelihood-based paternity inference in natural populations. Mol Ecol. 1998;7:639–655. doi: 10.1046/j.1365-294x.1998.00374.x. [DOI] [PubMed] [Google Scholar]
- 41.Wang JL. Sibship reconstruction from genetic data with typing errors. Genetics. 2004;166:1963–1979. doi: 10.1534/genetics.166.4.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalinowski ST, Wagner AP, Taper ML. ML-RELATE: a computer program for maximum likelihood estimation of relatedness and relationship. Mol Ecol Notes. 2006;6:576–579. [Google Scholar]
- 43.Fraley C, Raftery AE (2006; revised 2010) MCLUST version 3: an R package for normal mixture modeling and model-based clustering. Technical Report 504. Department of Statistics, University of Washington, Seattle.
- 44.Katsushima K, Nishida C, Yosida S, Kato M, Okanoya K, et al. A multiplex PCR assay for molecular sexing of the naked mole-rat (Heterocephalus glaber). Mol Ecol Resour. 2010;10:222–224. doi: 10.1111/j.1755-0998.2009.02742.x. [DOI] [PubMed] [Google Scholar]
- 45.Dakin EE, Avise JC. Microsatellite null alleles in parentage analysis. Heredity. 2004;93:504–509. doi: 10.1038/sj.hdy.6800545. [DOI] [PubMed] [Google Scholar]
- 46.Solmsen N, Johannesen J, Scradin C. Highly asymmetric fine scale structure between sexes of African striped mice and indication for condition dependent alternative male dispersal tactics. Mol Ecol. 2011;20:1624–1634. doi: 10.1111/j.1365-294X.2011.05042.x. [DOI] [PubMed] [Google Scholar]
- 47.Bennett NC, Jarvis JUM. The reproductive biology of the Cape mole-rat, Georychus capensis (Rodentia, Bathyergidae). J Zool. 1988;214:95–106. [Google Scholar]
- 48.Braude S. Dispersal and new colony formation in wild naked mole-rats: evidence against inbreeding as the system of mating. Behav Ecol. 2000;11:7–12. [Google Scholar]
- 49.Steinberg EK, Patton JL. Opportunities and constraints for evolutionary diversification. In: Life Underground: The Biology of Subterranean Rodents (eds. Lacey EA, Patton JL, Cameron GN), 257–296. University of Chicago Press, Chicago and London. 2000.
- 50.Lovegrove BG. The cost of burrowing by the social mole-rats (Bathyergidae) Cryptomys damarensis and Heterocephalus glaber: The role of soil moisture. Physiol Zool. 1989;62:449–469. [Google Scholar]
- 51.Thomas HG, Bateman PW, Le Comber SC, Bennett NC, Elwood RW, et al. Burrow architecture and digging activity in the Cape dune mole-rat. J Zool. 2009;279:277–284. [Google Scholar]
- 52.Herbst M, Bennett NC. Burrow architecture and burrowing dynamics of the endangered Namaqua dune mole-rat (Bathyergus janetta) (Rodentia: Bathyergidae). J Zool. 2006;270:420–428. [Google Scholar]
- 53.Heth G. Burrow patterns of the mole rat Spalaxehrenbergi in two soil types (terra-rossa and rendzina) in Mount Carmel, Israel. J Zool. 2009;217:39–56. [Google Scholar]
- 54.Nemec P, Cvekova P, Burda H, Benada O, Peichl L. Visual systems and the role of vision in subterranean rodents: diversity of retinal properties and visual system designs. In: Begall S, Burda H, Schleich CE (eds) Subterranean rodents: news from underground. Springer-Verlag, Berlin. 2007. pp. 129–160.
- 55.Narins PM, Reichman OJ, Jarvis JUM, Lewis ER. Seismic signal transmission between burrows of the Cape dune mole-rat, Georychus capensis. J Comp. 1992;Physiol170:13–21. doi: 10.1007/BF00190397. [DOI] [PubMed] [Google Scholar]
- 56.Zenuto RR, Fanjul MS, Busch C. Use of chemical communication by the subterranean rodent Ctenomys talarum (tuco tuco) during the breeding season. J Chem Ecol. 2004;30:2111–2126. doi: 10.1023/b:joec.0000048777.42945.e4. [DOI] [PubMed] [Google Scholar]
- 57.Clutton-Brock TH. Mammalian mating systems. Proc R Soc B. 1989;236:339–372. doi: 10.1098/rspb.1989.0027. [DOI] [PubMed] [Google Scholar]
- 58.Eberle M, Kappeler PM. Sex in the dark: determinants and consequences of mixed male mating tactics in Microcebus murinus, a small solitary nocturnal primate. Behav Ecol Sociobiol. 2004;57:77–90. [Google Scholar]
- 59.Baker RJ, Makova KD, Chesser RK. Microsatellites indicate a high frequency of multiple paternity in Apodemus (Rodentia). Mol Ecol. 1999;8:107. doi: 10.1046/j.1365-294x.1999.00541.x. [DOI] [PubMed] [Google Scholar]
- 60.Stockley P, Searle JB, Macdonald DW, Jones CS. Female multiple mating behaviour in the common shrew as a strategy to reduce inbreeding. Proc R Soc B. 1993;254:173–179. doi: 10.1098/rspb.1993.0143. [DOI] [PubMed] [Google Scholar]
- 61.Wolff JO, Macdonald DW. Promiscuous females protect their offspring. Trends Ecol Evol. 2004;19:127–134. doi: 10.1016/j.tree.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 62.Bishop JM, Jarvis JUM, Spinks AC, Bennett NC, O’Ryan C. Molecular insight into patterns of colony composition and paternity in the common mole-rat Cryptomys hottentotus hottentotus. Mol Ecol. 2004;13:1217–1229. doi: 10.1111/j.1365-294X.2004.02131.x. [DOI] [PubMed] [Google Scholar]
- 63.Bishop JM, O’Ryan C, Jarvis JUM. Social common mole-rats enhance outbreeding via extra-pair mating. Biol Letters. 2007;3:176–179. doi: 10.1098/rsbl.2006.0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jacobs DS, Jarvis JUM. No evidence for the work-conflict hypothesis in the eusocial naked mole-rat (Heterocephalus glaber). Behav Ecol Sociobiol. 1996;39:401–409. [Google Scholar]
- 65.Faulkes CG, Abbott DH, O’Brien HP, Lau L, Roy MR, et al. Micro- and macrogeographical genetic structure of colonies of naked mole-rats Heterocephalus glaber. Mol Ecol. 1997;6:615–628. doi: 10.1046/j.1365-294x.1997.00227.x. [DOI] [PubMed] [Google Scholar]
- 66.Burda H, Honeycutt RL, Begall S, Locker-Grutjen O, Scharff A. Are naked mole-rats eusocial and if so, why? Behav Ecol Sociobiol. 2000;47:293–303. [Google Scholar]
- 67.Hart L, Chimimba CT, Jarvis JUM, O’Riain J, Bennett NC. Craniometric sexual dimorphism and age variation in the South African cape dune mole-rat (Bathyergus suillus). J Mammal. 2007;88:657–666. [Google Scholar]
- 68.Fisher RA. The genetical theory of natural selection. - Clarendon Press, Oxford. 1930.
- 69.Hamilton WD. Extraordinary sex ratios. Science. 1967;156:477–488. doi: 10.1126/science.156.3774.477. [DOI] [PubMed] [Google Scholar]
- 70.Charnov EI. The Theory of Sex Allocation. Princeton, NJ: Princeton University Press. 1982.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary information for each locus including number of alleles (A), frequency of null alleles (Null), expected and observed heterozygosity (HO, HE), and FIS (Probability of significant deviation from Hardy-Weinberg equilibrium; P<0.05 = *, P<0.01 = **, P<0.001 = ***).
(DOCX)
Sire assignments for regions 1 (a) and 2 (b) across all models showing sire identity (sire-progeny distances in metres), probability of assignment, and number of mismatches between sire and progeny genotypes (NA denotes no sire assignment made).
(DOCX)
Plots showing the shape and number of either equal (E) or variable (V) volume components; (a) male length, the best BIC values were: V,2 (-6255), V,3 (-6264), E,2 (-6270), and (b) male mass, the best BIC values were:E,2 (-8853.623), V,2(-8858), E,3 (-8866).
(TIF)