Abstract
Background
Newborns suffer frequent infection and manifest impaired vaccine responses, motivating a search for neonatal vaccine adjuvants. Alum is a neonatal adjuvant, but may confer a Th2 bias. Toll-like receptor (TLR) agonists are candidate adjuvants, but human neonatal cord blood monocytes (Mos) demonstrate impaired Th1-polarizing responses to many TLR agonists due to plasma adenosine acting via cAMP. TLR8 agonists, including imidazoquinolines (IMQs) such as the small synthetic 3M-002, induce adult-level TNF from neonatal Mos, but the scope and mechanisms of IMQ-induced activation of neonatal Mos and Mo-derived dendritic cells (MoDCs) have not been reported.
Objectives
To characterize IMQ-induced activation of neonatal Mos and MoDCs.
Methods
Neonatal cord and adult peripheral blood Mos and MoDCs were cultured in autologous plasma; Alum- and TLR agonist-induced cytokines and co-stimulatory molecules were measured. TLR8 and inflammasome function were assayed using siRNA and western blotting/caspase-1 inhibitory peptide, respectively. The ontogeny of TLR8 agonist–induced cytokine responses was defined in Rhesus macaque whole blood ex vivo.
Results
IMQs were more potent and effective than Alum at inducing TNF and IL-1β from Mos. 3M-002 induced robust TLR pathway transcriptome activation and Th1-polarizing cytokine production in neonatal and adult Mos and MoDCs, signaling via TLR8 in an adenosine/cAMP- refractory manner. Newborn MoDCs displayed impaired LPS/ATP-induced caspase-1-mediated IL-1β production, but robust 3M-002-induced caspase-1-mediated inflammasome activation independent of exogenous ATP. TLR8-IMQs induced robust TNF and IL-1β in whole blood of Rhesus macaques at birth and infancy.
Conclusions
IMQ TLR8 agonists engage adenosine-refractory TLR8 and inflammasome pathways to induce robust Mo and MoDC activation and represent promising neonatal adjuvants.
Keywords: TLR8, Innate immunity, Neonate, Newborn, Alum, Adjuvant, Adenosine, IL-1β, Caspase-1, Inflammasome
INTRODUCTION
Each year over 2,000,000 newborns and infants worldwide die of infection (1, 2). Newborns express a distinct T-helper (Th) 2-polarized immune system at birth, potentially limiting the efficacy of crucial immunization efforts. Impairments in Th1-polarizing responses of neonatal antigen-presenting cells (APCs) limit neonatal immune responses (3). In response to most stimuli, neonatal monocytes (Mos) (4–6), monocyte-derived dendritic cells (MoDCs) (7), myeloid DCs (mDCs) (8) and plasmacytoid DCs (pDCs) (9) produce relatively low amounts of Th1-polarizing cytokines (10), including TNF, IFN-γ, interleukin (IL)-12p70, and IFN-α (11). DCs are specialized cells with very high intrinsic antigen-presenting activity important to vaccine responses (12). In this context, there is an unmet need for immunomodulators that can activate robust Th1-polarizing responses from neonatal APCs, to prevent and/or treat infection and allergies (13) or as vaccine adjuvants (14).
Currently, aluminum salt (Alum) is the major U.S. Food and Drug Administration-approved human vaccine adjuvant (15). Administered as part of vaccines to millions of people, it enhances antibody (Ab) production, effectively reducing morbidity and mortality. However, Alum-adjuvanted vaccines typically require multiple booster doses, fail to enhance cell-mediated immunity and may bias subsequent immune responses towards Th2 (16). Alum may exert its adjuvant effects in part via activation of the nucleotide-binding domain leucine-rich repeat-containing receptor containing a pyrin domain-3 (NLRP3) cytosolic inflammasome protein complex that mediates IL-1β and IL-18 production (17–19). Alum is given at birth as part of the hepatitis B vaccine, yet its activity towards primary neonatal leukocytes has not yet been reported with little known regarding expression/function of the inflammasome at birth (20).
Toll-like receptors (TLRs), sentinel components of innate immune defense, are activated by microbial products, synthetic compounds or endogenous danger signals (21). Engagement of TLRs activates APCs. Thus TLRs agonists can serve as stand-alone immune enhancing agents or as vaccine adjuvants (22, 23). The synthetic compound imiquimod (TLR7) is approved as a stand-alone topical agent for the treatment of human papilloma virus and certain skin cancers (24). A number of vaccines contain TLR agonists, including those against Haemophilus influenzae type b containing Neisseria-derived outer membrane proteins (TLR2) and Bacille Calmette-Guérin (Mycobacterium bovis; TLR2/4/8) (25), as well as certain hepatitis B vaccines that contain lipid A (TLR4) (12). Efforts are on going to identify TLR agonists that enhance adaptive immune responses of newborns and infants (26).
Impaired Th1 responses of human neonatal cord blood Mos and mDCs to agonists of TLRs 1-7 suggest that agonists of these TLRs may have limited immune adjuvant efficacy early in life (5, 6). Impairment of TLR2-mediated TNF production by human neonatal blood Mos is mediated in part by high extracellular concentrations of inhibitory adenosine in human neonatal cord blood plasma (27). Adenosine is an endogenous purine metabolite that acts via cognate adenosine receptors to induce intracellular production of cAMP, a secondary messenger that inhibits production of TNF and other Th1-polarizing cytokines, while maintaining/enhancing Th2 and anti-inflammatory cytokines (28, 29). Our prior studies of neonatal whole cord blood (CB), cord blood mononuclear cells (CBMCs) and Mos demonstrate that, in contrast to the impaired neonatal TNF response to agonists of TLRs 1-7, TLR8 agonists, including the IMQ resiquimod (R848; TLR7/8), induce adult-level TNF and IL-12p40/70 (30).
The TLR7, -8 and -9 subfamily is endosomal and activated by nucleic acids (31). Single-stranded viral RNAs (ssRNAs), such as those of influenza and human immunodeficiency viruses, are natural TLR7 and TLR8 agonists (32). Imidazoquinolines (IMQs) are small (<400 Da) synthetic antiviral compounds, which bear structural homology to the purine adenosine and activate mammalian leukocytes via TLR7 and/or TLR8 leading to MyD88-dependent NF-κB activation (24, 33). Whereas TLR7-agonist imiquimod activates Th1-polarizing responses from pDCs, including IFN-α, TLR8 agonists such as the small synthetic (Mr 243.33) IMQ 3M-002 activate Mos and mDCs to induce robust TNF production (34). Of note, IMQs engage the TLR pathway in a species-specific fashion: human and non-human primate TLR8 is activated by the same agonists (35), but murine TLR8 is divergent from human TLR in the expression of LRR repeats and therefore is not activated by agonists of human TLR8 (36). In addition to their TLR-mediated effects, IMQs also induce NLRP3 inflammasome-dependent IL-1β production in mice (37) as well as caspase-1 and IL-1β expression in human Mos and pDCs (38).
Our prior study demonstrated that TLR8 agonists might have unique stimulatory effects towards human neonatal mononuclear cells (39), but much remained unknown. In this study, we characterized the pattern of IMQ TLR8 agonist-induced activation of human neonatal Mos and MoDCs and the mechanisms underlying this activation. We show that IMQs induce, via TLR8- and caspase-1-mediated pathways refractory to inhibitory effects of adenosine/cAMP, Th1-type responses from neonatal APCs that exceed those induced by Alum or other TLR agonists. We also characterized the post-natal age range during which TLR8 agonists retain superior activity relative to other TLRs agonists Rhesus in macaque whole blood tested ex vivo. These results shed new light on the mechanisms governing neonatal Mo and MoDC responses, establish functional expression of the neonatal caspase-1/inflammasome as an adenosine-refractory pathway, and suggest that IMQ TLR8 agonists are promising candidate neonatal and infant immune adjuvants for the prevention and/or treatment of infection in the very young.
METHODS
See the Methods section in this article’s Online Repository (OR) for additional method details.
TLR agonists, assay reagents and Human Blood
TLR agonists included ultra-pure LPS from Salmonella minnesota, (TLR4; List Biological Laboratories, Campbell, CA) and IMQs 3M-013, R848 and 3M-002 (TLR7, TLR7/8 and TLR8 respectively; 3M Pharmaceuticals, St. Paul, MN). Human blood was collected under human experimentation guidelines of the U.S. Department of Health and Human Services.
Rhesus macaque blood
Rhesus macaque peripheral blood was collected in accordance with local institutional review board-approved animal study protocols.
Isolation of mononuclear cells and monocytes and Flow cytometry
Heparinized human blood was layered onto Ficoll-Hypaque gradients the cord or peripheral blood mononuclear cell (CBMC or PBMC, respectively) layer collected as previously described (40). Mos were isolated by negative selection. Flow cytometry analysis employing a MoFlo Legacy cytometer (DakoCytomation, Fort Collins, CO).
Differentiation of monocyte-derived dendritic cells (MoDCs)
Mos were cultured at 106/mL in RPMI (Gibco, Carlsbad, CA) with 10% fresh autologous plasma, supplemented with IL-4 and GM-CSF (each at 20 ng/ml) for 7 days. To assess caspase-1 activation, MoDCs were stimulated for 2 or 24 hours with a TLR agonist initially and 5 mM ATP (Sigma-Aldrich) added for 15 minutes prior to collection of supernatants (41, 42).
Measurement of pDC activation
Human newborn cord and adult peripheral blood was stimulated with TLR agonists at 37°C, with end-over-end rotation for 19 hours.
Quantitative RT-PCR array
Total RNA was isolated from lysates of TLR-stimulated Mos or DCs (QIAshredder spin column and RNeasy kit; Qiagen, Valencia, CA).
Cytokine, chemokine and cAMP measurement
Supernatants derived from human leukocyte stimulations were assayed by multiplex bead assay or where stated, by ELISA. cAMP was measured in lysates of neonatal Mos by competitive immunoassay as per manufacturer’s instructions (Thermo Scientific, Waltham, MA).
Small interfering (si) RNA
Employed siRNAs were targeted against TLR7 or TLR8 and a non-targeting siRNA (ON-TARGETplus SMARTpool, Dharmacon) to MoDCs on day 6/7 of cell culture using electroporation (human DC-electroporation program, Amaxa Nucleofection System, Basel, Switzerland).
Caspase-1 western blotting
Supernatants of TLR agonist-stimulated MoDCs (106/mL) cell lysates were collected for protein determination (BCA protein assay, Pierce, Waltham, MA). Clarified lysate was subjected to acetone precipitation at −20°C for 90 minutes, SDS-PAGE and western blot (43).
Statistical analysis and graphics
Data were analyzed using Prism for MacIntosh v. 5.0b (GraphPad Software Inc.; San Diego, CA). Data represent means ± SEM. For normal sample sets, two-tailed t-test or One-way ANOVA with Bonferroni post-test were applied as appropriate. Non-normal sample sets were analyzed by Wilcoxon signed-rank test or by Mann-Whitney test as appropriate. p values < 0.05 were considered significant.
RESULTS
Imidazoquinolines are more potent and efficacious than Alum in inducing IL-1β and TNF from human neonatal and adult monocytes
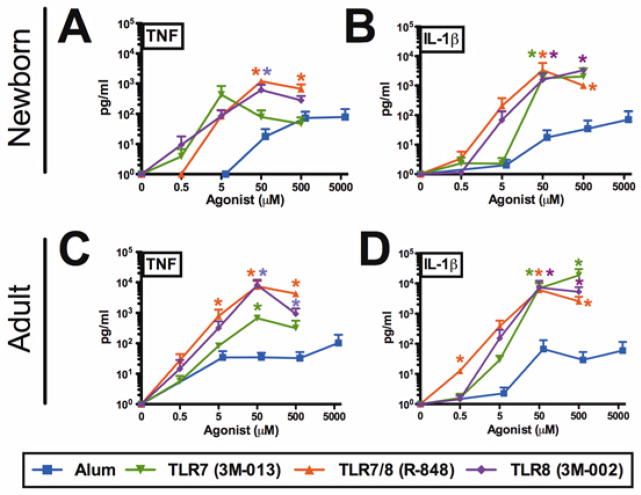
We compared the in vitro bioactivity of IMQs to that of Alum. Human newborn cord and adult peripheral blood-derived Mos were cultured in autologous plasma and stimulated with Alum, 3M-013 (TLR7), R848 (TLR7/8) or 3M-002 (TLR8). Compared to Alum, IMQs were ~10–100 fold more potent and effective at inducing both TNF and IL-1β from both newborn and adult Mos, inducing ~1,000 to 10,000 pg/mL of these cytokines at 5–50 μM. In marked contrast, Alum failed to induce comparable cytokine concentrations even at 500–5,000 μM (Fig 1).
FIG 1. TLR7/8 and TLR8 agonists are more potent and effective than Alum in inducing TNF and IL-1β from human newborn and adult monocytes.
A, and B, newborn or C and D, adult stimulated monocytes for 4 hrs (TNF) or 18 hrs (IL-1β) and supernatants were assayed by ELISA. n = 3–4; * P ≤0.05 (Mann-Whitney test comparing TLR agonist to Alum).
Imidazoquinoline TLR8 agonist induces robust expression of a TLR transcriptome and Th1-polarizing cytokine/chemokines by human cord blood monocytes
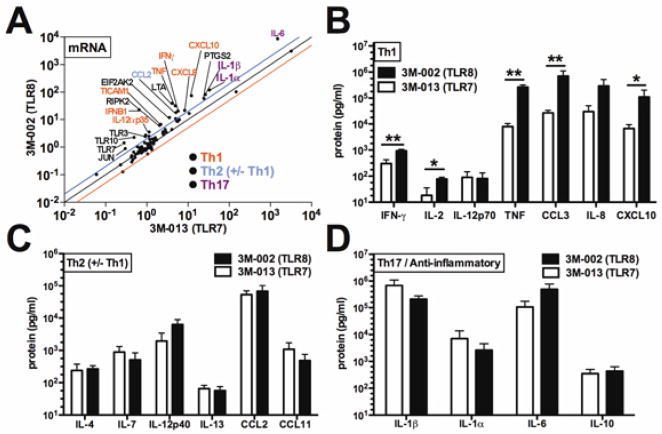
We compared human newborn Mos responses to 3M-013 with those to 3M-002 (Fig 2). We selected the agonist concentrations required to achieve TNF production per our prior studies (30), and a stimulation time-point (4h) based on kinetic data (and unpublished observations) (5). In comparison to buffer control, 3M-002 greatly increased levels of mRNAs encoding IL-6 (8603-fold change, p < 0.001), pro-IL-1β (82-fold change, p < 0.001), IFN-β1 (21-fold change p < 0.01), IFN-γ(21- fold change, p < 0.05) and TNF (32-fold change, p < 0.0001) (data not shown). 3M-002 induced significantly greater overall increases in TLR pathway mRNAs in newborn Mos than did 3M-013, with a mean log-fold difference in mRNA levels of +0.1862 (p < 0.0001; Fig 2, A). Compared to 3M-013, 3M-002 induced greater expression of mRNAs encoding Th1-polarizing cytokines/chemokines, including CXCL10 (IP-10), IFN-γ, TNF, IFN-β1, IL-12α (p35) as well as TICAM-1 (Fig 2, A). 3M-002 also induced greater Th1-polarizing responses than MALP (TLR2/6), with a mean log-fold difference in mRNA levels of +0.2156 (p < 0.0001), including greater induction of TNF, IFN-β1, and lymphotoxin α (Fig E1). Effects of 3M-002 and 3M-013 on neonatal Mo cytokine expression were further compared at the protein level. Consistent with mRNA results, 3M-002 induced significantly greater protein concentrations of Th1-polarizing cytokines/chemokines including, IL-2, TNF, IFN-γ CXCL10 and CCL3 (MIP-1α) (Fig 2, B). In contrast, cytokines/chemokines with Th2 (±Th1)-polarizing potential (including IL-4, IL-7, IL-12p40 and IL-13), Th17-polarizing potential (IL-1β) or anti-inflammatory-polarizing potential (IL-6, IL-10) were induced to similar levels by both agonists (Fig 2, C-D). Similarly, when compared to MALP (TLR2/6), 3M-002 induced significantly greater concentrations of Th1-polarizing cytokines such as IL-12p70, TNF, IFN-γ and CXCL8 (IL-8) (Fig E1, B). With respect to Th2 (±Th1), Th17-polarizing, or anti-inflammatory cytokines/chemokines, 3M-002 induced significantly greater concentrations of IL-1α, IL-1β, IL-4 and CCL11 (eotaxin) and also trended towards higher concentrations of the other cytokines tested (Fig E1, C-D).
FIG 2. The TLR8 agonist 3M-002 induces greater TLR pathway mRNA up-regulation and Th1-polarizing cytokines/chemokines from neonatal Mos than a TLR7 agonist.
Monocytes (5 × 107 cells/mL) stimulated with indicated agonist (50 μM), 4 h. A, Agonist-induced mRNA fold change relative to control (colored lines indicate 2-fold change). B-D; Th1, Th2 (±Th1), Th17 and anti-inflammatory proteins. n = 3–6; * P < 0.05, ** P < 0.01.
Additionally we characterized siRNA-mediated inhibition of TLR8 gene expression in human neonatal Mos (see results section and Fig E2 in this article’s Online Repository), which to our knowledge is the first report of the use of siRNA technology in primary neonatal APCs. 3M-002 also induced greater TNF, IFN-γ and IL-12p70 than Alum in human newborn MoDCs (see results section and Fig E3 in this article’s Online Repository). 3M-002 and R848 demonstrated greater activation of neonatal MoDCs than 3M-013 with respect to up-regulation of CD40, 80, 86, and 83 as well as IL-12p70 production. As TLR7-mediated pDC activation is important for enhancing Th1 responses, including cross-presentation to CD8-bearing T cells (44), it is notable that R848 also induced robust neonatal pDC activation in whole blood as measured by IFN-α production and up-regulation of CD40, to adult-like levels (see OR results section and Fig E4 in this article’s Online Repository).
TLR8 agonist 3M-002 is refractory to the inhibitory adenosine-cAMP axis
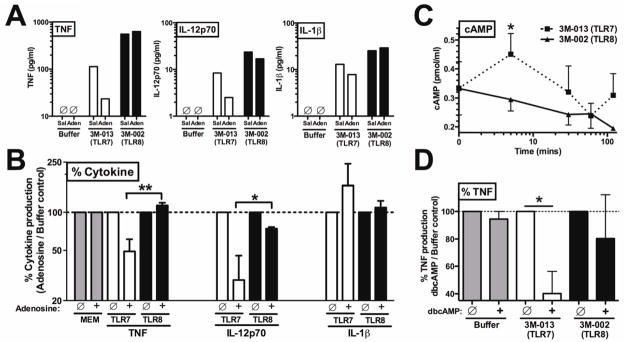
To further characterize the mechanisms by which IMQs activate neonatal Mos, whose activity can be limited by adenosine acting via cAMP production (27), we assessed the relative effects of adenosine on TLR7- vs. TLR8-mediated TNF and IL-12p70 production by newborn Mos cultured in autologous plasma. Pre-incubation of newborn Mos with adenosine inhibited 3M-013-induced TNF and IL-12p70 production by up to ~70% (Fig 3, A). In contrast, 3M-002 was refractory to the inhibitory effect of adenosine. Adenosine did not reduce IL-1β production induced by either compound (Fig 3, A), suggesting that the inflammasome pathway may be refractory to adenosine inhibition. Analyzed as normalized percentage, 3M-002 was significantly more refractory to adenosine inhibition than to 3M-013 (Fig 3, B). As adenosine can mediate inhibitory effects via induction of cAMP (45), we measured intracellular cAMP concentrations in newborn Mos exposed to TLR7 or TLR8 agonists. Exposure of neonatal Mos to 3M-002 resulted in significantly less early (5 min, p < 0.05) cAMP accumulation than did exposure to 3M-013 (Fig 3, C). We determined whether pharmacologic enhancement of intracellular cAMP concentrations using the cell-permeable molecule dibutyryl-cAMP (db-cAMP) affected TLR-mediated TNF production. Whereas addition of db-cAMP inhibited TLR7 agonist-induced TNF production (p < 0.05), TLR8 agonist-induced TNF was largely refractory (Fig 3, D). Overall, the standard errors of the mean for these conditions were relatively large, likely reflecting study of primary cells in autologous plasma, rather than cell lines in defined culture medium, but the effects described were statistically significant. Thus stimulation 3M-002 not only resulted in less cAMP accumulation in neonatal Mos, but also engaged a pathway for TNF production that was relatively less sensitive to intracellular cAMP concentrations.
FIG 3. TLR8 agonists are refractory to inhibitory adenosine/cAMP and associated with lesser cAMP accumulation in newborn Mos.
A, Monocytes, to which adenosine (10 μM) or saline (Sal) added prior to stimulation with agonists (50 μM) for 2 hrs (representative of 3-6 experiments). B, % cytokine. C, cAMP concentrations in agonist treated monocytes. D, 30 min db-cAMP (10 μM) treated monocytes, 4 hr stimulation. n =3–6; * P < 0.05, ** P < 0.01.
Newborn MoDCs demonstrate impaired caspase-1-dependent IL-1β production in response to LPS/ATP
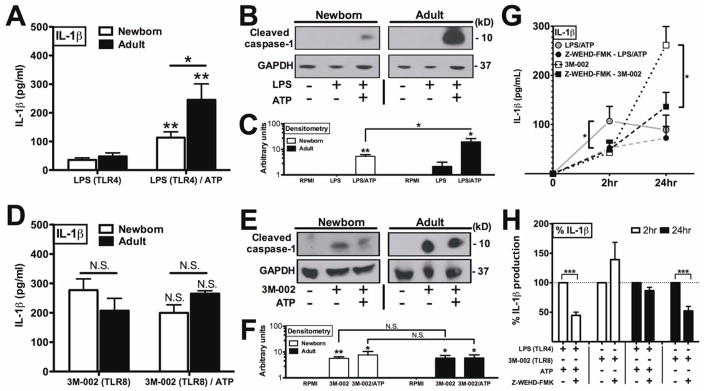
Little is known about expression or function of the inflammasome in neonatal leukocytes. Newborn and adult MoDCs were stimulated with LPS (TLR4) followed by ATP, a combination known to activate inflammasome-dependent IL-1β production in adult MoDCs (19, 41). Of note, neither buffer alone nor ATP alone induced IL-1β production. When exposed to LPS (alone) for 2 hours, both newborn and adult MoDCs produced low levels of IL-1β (Fig 4, A and active) caspase-1 (Fig 4, B and C). Addition of ATP to LPS-stimulated MoDCs significantly increased IL-1β production for both newborn and adult MoDCs (Fig 4, A, p < 0.01), but to a significantly lesser extent in newborn as compared to adult MoDCs (Fig 4, A, p < 0.05). Accordingly, western blotting of lysates derived from LPS/ATP treated MoDCs demonstrated diminished production of the active 10 kDa caspase-1 fragment in neonatal as compared to adult MoDCs (Fig 4, B and C). These results suggest a limitation in neonatal inflammasome activation with respect to certain stimuli.
FIG 4. Newborn MoDCs display diminished LPS/ATP-induced but robust IMQ-induced caspase-1 activation and IL-1β production.
Newborn and adult MoDCs stimulated with LPS (2 hrs) (A, B, C) or 3M-002 (24 hrs) (D, E, F), plus 5 mM ATP as indicated. Supernatant assayed IL-1β (ELISA) (A, D), cell lysates caspase-1 by western blotting/densitometry (B, C, E, F). C, F representative of n = 3; A, D n = 4–6, Asterisk denotes comparison between treatment conditions. Line-bar indicates comparison between groups (newborn and adult). G, Caspase-1 inhibitor Z-WEHD-FMK pretreatment of neonatal MoDCs treated as above. H, IL-1β data from G shown as %. n = 4–6; * P < 0.05, ** P < 0.01, *** P < 0.001.
3M-002 activates robust caspase 1-dependent IL-1β production in neonatal MoDCs independent of exogenous ATP
We next evaluated the ability of 3M-002 to activate the neonatal inflammasome. In contrast to LPS, which required addition of exogenous ATP to boost IL-1β production, 3M-002, when added alone, induced robust IL-1β production in both adult MoDCs and neonatal MoDCs. Exogenously added ATP did not further increase 3M-002-induced production of IL-1β (Fig 4, D). Analysis of inflammasome pathway mRNA expression indicated that there was no significant difference of in amounts of 3M-002 induced compared to LPS induced pro-IL-1β mRNA (Fig E5), suggesting that differences in IL-1β production were not due to differences at the mRNA level. Accordingly, in contrast to LPS (Fig 4, B, C), 3M-002 induced caspase-1 activation in both newborn and adult MoDCs independently of exogenous ATP as assessed by western blotting (Fig 4, E, F). In contrast to LPS/ATP induction of IL-1β, which peaked at 2 hours, 3M-002-induced IL-1β demonstrated distinct kinetics with a marked increase in IL-1β production at 24 hrs of stimulation (Fig. E6, A). Independence of 3M-002-induced IL-1β production from exogenous ATP across this timeframe was also evident when the data were expressed as %IL-1β production in the ATP-treated condition relative to that induced without ATP (Fig. E6, B). To further characterize the action of 3M-002 on neonatal MoDCs, we determined whether 3M-002-induced IL-1β production was caspase-1-dependent. To this end, neonatal MoDCs were pre-incubated with or without the caspase-1-selective antagonist Z-WEHD-FMK prior to stimulation (Fig 4, G). Z-WEHD-FMK inhibited both LPS/ATP- and 3M-002 (alone)-induced IL-1β, an effect that was also evident when analyzed as % IL-1β production (Fig. 4, H, p < 0.001). Effects of Z-WEHD-FMK were specific to IL-1β production as TNF production was unaffected (data not shown).
Imidazoquinoline TLR8 agonists induce robust cytokine and co-stimulatory responses in blood from newborn and infant Rhesus macaques
We next characterized the ontogeny of TLR8 agonist activity towards peripheral blood leukocytes during the first weeks of life by employing whole blood derived from newborn and infant Rhesus macaques, a species that expresses TLR8 that is functionally similar to that of humans (35, 46, 47). Rhesus macaque blood was drawn at birth (newborn cord), weekly basis for the first three weeks of life (peripheral blood from same animals) as well as from dam and non-dam adults. We compared the ability of MALP, 3M-013, R848 and 3M-002 to induce TNF and IL-1β production ex vivo. In marked contrast to the TLR2 and TLR7 (only) agonists, R848 and 3M-002 demonstrated greater TNF induction (Fig E7, A-D), inducing cytokine levels comparable to those in adult blood. Similarly, although demonstrating an apparent age-dependent maturation in production from birth (cord) to 1 week of life, R848 and 3M-002 induced robust IL-1β production, at least as great as adult levels, at weeks 1-3 of life (Fig E7. EH). R848 also demonstrated efficacy in inducing up-regulation of CD40 in both infant (1–4 months) and adult Rhesus macaque mDCs in whole blood (Fig E4, D).
DISCUSSION
In this study, we characterized the scope and mechanisms of neonatal Mo and MoDC activation by IMQ TLR7 and/or -8 agonists. In neonatal Mos, 3M-002 was more effective than 3M-013 at inducing mRNA and protein expression of Th1-polarizing cytokines, suggesting that IMQ TLR7/8 agonists may offer greater adjuvant potency and efficacy than Alum. Since 3M-002-induced TNF production from neonatal primary MoDCs was at least in part TLR8-dependent, we further characterized the mechanism of TLR8-induced APC activation and cytokine production in neonatal Mos by assessing the relative sensitivity of TLR7 and TLR8 agonists to the inhibitory adenosine-cAMP axis. Adenosine modulates TLR-mediated cytokine production (28) and DC activity (48) and can differentially regulate DC-primed Th cell responses, increasing Th2 responses in atopic study subjects while reducing Th1 and increasing Treg activity in non-atopic donors (49). High neonatal plasma concentrations of adenosine, coupled with sensitivity of neonatal mononuclear cells to adenosine-induced intracellular cAMP accumulation, suppresses the ability of neonatal Mos to produce pro-inflammatory/Th1-polarizing cytokines (11). Remarkably, neonatal Mos stimulated with TLR8 agonists were refractory to the inhibitory effects of adenosine (Fig 3) and accumulated less cAMP than those exposed to a TLR7 agonist (Fig 3, C). TLR8 agonists were also refractory to pharmacologic enhancement of cAMP using cell-permeable db-cAMP (Fig 3, D). As cAMP is downstream of adenosine in an inhibitory pathway, this suggests that TLR8 activation engages a cAMP refractory pathway, potentially as an adenosine receptor antagonist (50). Collectively, these data suggest that an IMQ TLR8 agonist can act refractory to the adenosine/cAMP axis by: 1) reducing intracellular cAMP accumulation and 2) engaging pathways insensitive to cAMP (Fig 3).
In addition to the TLR-NF-κB pathway, increasing attention has focused on the inflammasome’s role in immune responses (51). Inflammasome-mediated IL-1β production may contribute to vaccine responses by enhancing Ab production (17). Little is known regarding the effects of IMQs on inflammasome activation in primary human leukocytes, with published studies largely confined to adult cells cultured in serum-free media or in fetal calf serum (38, 52, 53) that have largely focused on IL-1β production, with the exception of one study of caspase-1 activation in human adult Mos and pDCs (38). We have compared for the first time the action of Alum and IMQs in primary human newborn and adult MoDCs cultured in fresh autologous human plasma. Of note, in contrast to the production of TNF and IL-12p70, both TLR7- and TLR8-mediated Mo IL-1β were largely refractory to adenosine, to our knowledge the first time the effects of adenosine on the inflammasome pathway have been evaluated.
Little is known about the functional expression of the inflammasome at birth. Neonatal CBMCs demonstrate lesser LPS-induced IL-1β than adult PBMC (6), but there are no published reports of caspase activity in neonatal leukocytes. Here, we demonstrated for the first time that IMQs are effective inducers of IL-1β in human cord blood-derived Mos and MoDCs. 3M-002 and R848 were ~10-100-fold more potent and effective inducers of IL-β in human newborn and adult Mos than Alum, whose adjuvant properties may in part be inflammasome-dependent (17), though additional mechanisms are under study (54). We demonstrate for the first time the functional expression of a caspase-1 inflammasome in human newborn MoDCs. Primary human neonatal MoDCs demonstrated TLR agonist-induced expression of pro-IL-1β mRNA, as well as caspase-1 activation and IL-1β protein production. Within our limited cohort of study subjects, we discovered diminished LPS/ATP-induced caspase-1 activation and IL-1β production in neonatal MoDCs compared to adult MoDCs, indicating a stimulus-specific impairment in caspase-1/inflammasome pathway at birth.
In contrast to Alum’s limited ability to activate primary MoDCs in vitro, IMQs activated caspase-1 and induced IL-1β production. Similarly to LPS, 3M-002-induced IL-1β protein production was caspase-1-dependent as it was sensitive to the specific inhibitor Z-WEHD-FMK. However, in contrast to LPS, 3M-002 demonstrated: a) distinct kinetics with a marked increase in IL-1β production at 24 hrs of stimulation and b) independence from ATP. 3M-002 may require time to diffuse into the endosomal compartment to activate endosomal TLR8 (needed for pro-IL-1β mRNA). In contrast, the combination of LPS/ATP results in rapid surface activation of TLR4-NF-κB pathway and exogenous ATP inflammasome activation respectively. The ability of IMQs to induce IL-1β without exogenous ATP, directly through ligand-receptor interaction or through yet to be determined secondary effects, may contribute to relatively high IMQ-induced IL-1β. In addition, the ability of TLR8-signaling IMQs to trigger both NF-κB and inflammasome pathways in an adenosine- and exogenous ATP-independent manner (see model in Fig 5), respectively, may contribute to the ability of these agents to consistently induce TNF and IL-1β responses not only at birth but throughout infancy as demonstrated in Rhesus macaque blood tested ex vivo (see OR results section and Fig E7 in this article’s Online Repository). Further investigation of crosstalk in the NF-kB and inflammasome signaling pathways in early life is warranted, including the study of combinatorial effects of Alum and TLR agonists.
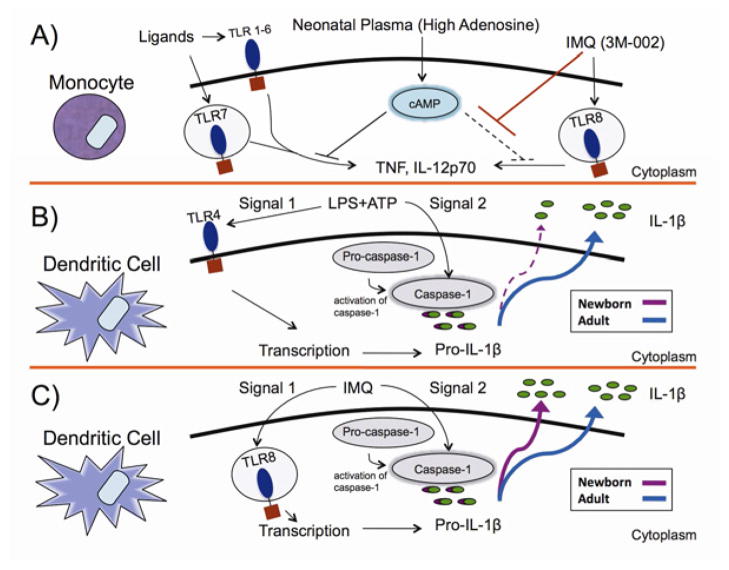
FIG 5. Proposed mechanisms of Imidazoquinoline TLR8 agonist activation of human newborn monocytes and DCs via adenosine-refractory and caspase-1-dependent pathways.
3M-002 agonists are refractory to inhibitory effects of the adenosine/cAMP pathway, which skews neonatal immunity against Th1-polarizition (A), can overcome impaired IL-1β responses of neonatal DCs to LPS/ATP (B) independently of exogenous ATP (C).
Relatively high TLR8 agonist-induced TNF and IFN-γ induction was also evident in a recent study of whole blood derived from human infants in The Gambia (55). IMQs carry substantial potential as vaccine adjuvants that can be formulated either as covalently conjugated to antigen (56), or as topical adjuvants with subsequent injection of antigen (57) to enhance antigen-specific responses (58). Our findings may thus have translational implications. 3M-002-induced IFN-γ production is of particular interest given the impaired ability of newborn Mos/APCs to produce this key Th1-poalirzing cytokine in response to many stimuli (59). TLR8 IMQ-induced IL-1β production is also notable, given that inflammasome-dependent production of this cytokine may contribute to the ex vivo effects of adjuvants (17, 37). Future studies will characterize how biochemically distinct TLR8 agonists, such as ssRNAs, induced IL-1β production, and in combination with agonists of other TLRs, may induce synergistic maturation of neonatal and adult DCs (52, 53) that may increase antigen-specific responses in vivo (60).
As neonatal APC responses to many stimuli are impaired and/or skewed (6, 11), our work on agents that potentially induce Th1-polarizing responses from neonatal APCs is novel and of potential translational importance, but further work remains to be done. We demonstrate that IMQs activate neonatal APCs in vitro to produce cytokines with Th1-polarizing properties (e.g, IFN-γ , IL-12p70). Future work should assess whether this activity extends to actual DC-T cell interactions. In vitro modeling of the effects of insoluble Alum crystals may incompletely reflect the activity of Alum in vivo, and additional approaches such as animal models may provide further insight in comparing the adjuvant activities of Alum and IMQs.
Identifying novel neonatal vaccine adjuvants is key to the development of more effective vaccines against common infections of neonates and infants (61). We demonstrated that imidazoquinoline TLR8 agonists are effective inducers of TNF and IL-1β in both cord blood and infant peripheral blood of Rhesus macaques. These data demonstrate that TLR8 agonists maintain greater immunostimulatory properties not only at birth (cord blood) but also in peripheral blood obtained during infancy, an important phase of susceptibility and immunization schedules. Future translational studies should evaluate the safety and efficacy of vaccine formulations containing TLR7/8 agonists in newborns animals, focusing on species such as Rhesus macaques that express TLR8 with structural and functional similarities to human (56). Given the high global burden of infections in newborns and infants (20, 62) and the unmet medical need for more effective neonatal vaccines that target individuals at birth, the most frequent point of healthcare contact (61, 63), this approach now merits timely evaluation.
Supplementary Material
Clinical Implications.
Imidazoquinoline TLR8 agonists are more effective than the conventional vaccine adjuvant Alum at activating human neonatal antigen-presenting cells (APCs) and are promising candidate adjuvants to enhance neonatal vaccine responses.
Acknowledgments
We thank Drs. Christine Palmer, Matthew Pettengill, Adi Reske, Isaac Kohane, for expert technical advice and helpful discussions. Drs. Michael R. Wessels, Raif Geha, Richard Malley, and Christopher Wilson are acknowledged for helpful conversations and mentorship. Amity M. Paye, Amy Li, Sibel Guzel, Erika Levis, Emily Babendreier, Kamila Naxerova, Christy Mancuso, and Elizabeth Boush provided expert technical assistance. The assistance of the labor and delivery staff at The Brigham & Women’s Hospital is gratefully acknowledged.
Abbreviations used
- Ab
antibody
- Alum
Aluminum hydroxide and magnesium hydroxide (Imject Alum)
- APC
Antigen-presenting cells
- ATP
Adenosine-5'-triphosphate
- BCA
bicinchoninic acid
- cAMP
Cyclic adenosine monophosphate
- DCs
dendritic cells
- db-cAMP
dibutyryl-cAMP
- IFN
Interferon
- IL
Interleukin
- IMQ
Imidazoquinoline
- LPS
Lipopolysaccharide
- MALP
Macrophage-activating lipopeptide-2
- mDCs
Myeloid dendritic cells
- Mos
Monocytes
- MoDCs
Monocyte-derived DCs
- NALP3
Nacht domain leucine-rich repeat and PYD-containing protein 3 (also known as Nlrp3)
- pDCs
Plasmacytoid DCs
- Sal
Saline
- siRNA
Small interfering (si) Ribonucleic acid (RNA)
- Th
T-helper
- TLR
Toll-like receptor
- TNF
Tumor necrosis factor
Footnotes
Declaration of all sources of funding: This project was supported by grants to OL from the National Institutes of Health RO1 AI067353-01A1 and Administrative Supplement 3R01AI067353-05S1, The Patterson Trust, and by Grand Challenges Explorations Grant 53278, and Global Health Grant OPPGH5284 from The Bill & Melinda Gates Foundation. Reagent support was provided by 3M Pharmaceuticals. VJP was supported by an American Heart Association Postdoctoral Fellowship.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegrist CA. The challenges of vaccine responses in early life: selected examples. J Comp Pathol. 2007 Jul;137(Suppl 1):S4–9. doi: 10.1016/j.jcpa.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 2.UNICEF. The State of the World’s Children Special Edition: Celebrating 20 Years of the Convention on the Rights of the Child. 2009. [Google Scholar]
- 3.Willems F, Vollstedt S, Suter M. Phenotype and function of neonatal DC. Eur J Immunol. 2009 Jan;39(1):26–35. doi: 10.1002/eji.200838391. [DOI] [PubMed] [Google Scholar]
- 4.Yan SR, Qing G, Byers DM, Stadnyk AW, Al-Hertani W, Bortolussi R. Role of MyD88 in diminished tumor necrosis factor alpha production by newborn mononuclear cells in response to lipopolysaccharide. Infection & Immunity. 2004 Mar;72(3):1223–9. doi: 10.1128/IAI.72.3.1223-1229.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levy O, Zarember KA, Roy RM, Cywes C, Godowski PJ, Wessels MR. Selective impairment of TLR-mediated innate immunity in human newborns: neonatal blood plasma reduces monocyte TNF-alpha induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod, but preserves the response to R-848. J Immunol. 2004 Oct 1;173(7):4627–34. doi: 10.4049/jimmunol.173.7.4627. [DOI] [PubMed] [Google Scholar]
- 6.Kollmann TR, Crabtree J, Rein-Weston A, Blimkie D, Thommai F, Wang XY, et al. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol. 2009 Dec 1;183(11):7150–60. doi: 10.4049/jimmunol.0901481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langrish CL, Buddle JC, Thrasher AJ, Goldblatt D. Neonatal dendritic cells are intrinsically biased against Th-1 immune responses. Clin Exp Immunol. 2002 Apr;128(1):118–23. doi: 10.1046/j.1365-2249.2002.01817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darmochwal-Kolarz D, Rolinski J, Buczkowski J, Tabarkiewicz J, Leszczynska-Gorzelak B, Zych I, et al. CD1c(+) immature myeloid dendritic cells are predominant in cord blood of healthy neonates. Immunol Lett. 2004 Jan 30;91(1):71–4. doi: 10.1016/j.imlet.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 9.De Wit D, Olislagers V, Goriely S, Vermeulen F, Wagner H, Goldman M, et al. Blood plasmacytoid dendritic cell responses to CpG oligodeoxynucleotides are impaired in human newborns. Blood. 2004 Feb 1;103(3):1030–2. doi: 10.1182/blood-2003-04-1216. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen M, Leuridan E, Zhang T, De Wit D, Willems F, Van Damme P, et al. Acquisition of adult-like TLR4 and TLR9 responses during the first year of life. PLoS One. 5(4):e10407. doi: 10.1371/journal.pone.0010407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levy O. Innate immunity of the newborn: basic mechansims and clinical correlates. Nature Reviews Immunology. 2007;7:379–90. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 12.van Duin D, Medzhitov R, Shaw AC. Triggering TLR signaling in vaccination. Trends Immunol. 2006 Jan;27(1):49–55. doi: 10.1016/j.it.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Belderbos M, Levy O, Bont L. Neonatal innate immunity in allergy development. Curr Opin Pediatr. 2009 Dec;21(6):762–9. doi: 10.1097/MOP.0b013e3283325e3a. [DOI] [PubMed] [Google Scholar]
- 14.Philbin VJ, Levy O. Developmental biology of the innate immune response: implications for neonatal and infant vaccine development. Pediatr Res. 2009 May;65(5 Pt 2):98R–105R. doi: 10.1203/PDR.0b013e31819f195d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol. 2009 Apr;9(4):287–93. doi: 10.1038/nri2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van den Biggelaar AH, Richmond PC, Pomat WS, Phuanukoonnon S, Nadal-Sims MA, Devitt CJ, et al. Neonatal pneumococcal conjugate vaccine immunization primes T cells for preferential Th2 cytokine expression: a randomized controlled trial in Papua New Guinea. Vaccine. 2009 Feb 25;27(9):1340–7. doi: 10.1016/j.vaccine.2008.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008 May 21;453(7198):1122–7. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kool M, Petrilli V, De Smedt T, Rolaz A, Hammad H, van Nimwegen M, et al. Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol. 2008 Sep 15;181(6):3755–9. doi: 10.4049/jimmunol.181.6.3755. [DOI] [PubMed] [Google Scholar]
- 19.Li H, Nookala S, Re F. Aluminum hydroxide adjuvants activate caspase-1 and induce IL-1beta and IL-18 release. J Immunol. 2007 Apr 15;178(8):5271–6. doi: 10.4049/jimmunol.178.8.5271. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez-Schmitz G, Levy O. Development of newborn and infant vaccines. Sci Transl Med. 2011 Jul 6;3(90):90ps27. doi: 10.1126/scitranslmed.3001880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monie TP, Bryant CE, Gay NJ. Activating immunity: lessons from the TLRs and NLRs. Trends Biochem Sci. 2009 Nov;34(11):553–61. doi: 10.1016/j.tibs.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 22.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. Oct 29;33(4):492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomai MA, Vasilakos JP. TLR-7 and -8 agonists as vaccine adjuvants. Expert Rev Vaccines. 2011 Apr;10(4):405–7. doi: 10.1586/erv.11.26. [DOI] [PubMed] [Google Scholar]
- 24.Miller RL, Meng TC, Tomai MA. The antiviral activity of Toll-like receptor 7 and 7/8 agonists. Drug News Perspect. 2008 Mar;21(2):69–87. doi: 10.1358/dnp.2008.21.2.1188193. [DOI] [PubMed] [Google Scholar]
- 25.Davila S, Hibberd ML, Hari Dass R, Wong HE, Sahiratmadja E, Bonnard C, et al. Genetic association and expression studies indicate a role of toll-like receptor 8 in pulmonary tuberculosis. PLoS Genet. 2008 Oct;4(10):e1000218. doi: 10.1371/journal.pgen.1000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanswal S, Katsenelson N, Selvapandiyan A, Bram RJ, Akkoyunlu M. Deficient TACI expression on B lymphocytes of newborn mice leads to defective Ig secretion in response to BAFF or APRIL. J Immunol. 2008 Jul 15;181(2):976–90. doi: 10.4049/jimmunol.181.2.976. [DOI] [PubMed] [Google Scholar]
- 27.Levy O, Coughlin M, Cronstein B, Roy RM, Desai A, Wessels MR. The adenosine system selectively inhibits TLR-mediated TNF-alpha production in the human newborn. J Immunol. 2006;177:1956–66. doi: 10.4049/jimmunol.177.3.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Power Coombs MR, Belderbos ME, Gallington LC, Bont L, Levy O. Adenosine modulates Toll-like receptor function: basic mechanisms and translational opportunities. Expert Rev Anti Infect Ther. 2011 Feb;9(2):261–9. doi: 10.1586/eri.10.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasko G, Pacher P, Deitch EA, Vizi ES. Shaping of monocyte and macrophage function by adenosine receptors. Pharmacol Ther. 2007 Feb;113(2):264–75. doi: 10.1016/j.pharmthera.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levy O, Suter EE, Miller RL, Wessels MR. Unique efficacy of Toll-like receptor 8 agonists in activating human neonatal antigen-presenting cells. Blood. 2006;108:1284–90. doi: 10.1182/blood-2005-12-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blasius AL, Beutler B. Intracellular toll-like receptors. Immunity. 2010 Mar 26;32(3):305–15. doi: 10.1016/j.immuni.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 32.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004 Mar 5;303(5663):1526–9. doi: 10.1126/science.1093620. [see comment] [DOI] [PubMed] [Google Scholar]
- 33.Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nature Immunology. 2002;3(2):196–200. doi: 10.1038/ni758. [comment] [DOI] [PubMed] [Google Scholar]
- 34.Philbin VJ, Levy O. Immunostimulatory activity of Toll-like receptor 8 agonists towards human leucocytes: basic mechanisms and translational opportunities. Biochem Soc Trans. 2007 Dec;35(Pt 6):1485–91. doi: 10.1042/BST0351485. [DOI] [PubMed] [Google Scholar]
- 35.Wille-Reece U, Flynn BJ, Lore K, Koup RA, Kedl RM, Mattapallil JJ, et al. HIV Gag protein conjugated to a Toll-like receptor 7/8 agonist improves the magnitude and quality of Th1 and CD8+ T cell responses in nonhuman primates. Proc Natl Acad Sci U S A. 2005 Oct 18;102(42):15190–4. doi: 10.1073/pnas.0507484102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004 Mar 5;303(5663):1526–9. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 37.Kanneganti TD, Ozoren N, Body-Malapel M, Amer A, Park JH, Franchi L, et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006 Mar 9;440(7081):233–6. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 38.Hurst J, Prinz N, Lorenz M, Bauer S, Chapman J, Lackner KJ, et al. TLR7 and TLR8 ligands and antiphospholipid antibodies show synergistic effects on the induction of IL-1beta and caspase-1 in monocytes and dendritic cells. Immunobiology. 2009;214(8):683–91. doi: 10.1016/j.imbio.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 39.Levy O, Suter EE, Miller RL, Wessels MR. Unique efficacy of Toll-like receptor 8 agonists in activating human neonatal antigen-presenting cells. Blood. 2006 Aug 15;108(4):1284–90. doi: 10.1182/blood-2005-12-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levy O, Zarember KA, Roy RM, Cywes C, Godowski PJ, Wessels MR. Selective impairment of Toll-like receptor-mediated innate immunity in human newborns: Neonatal blood plasma reduces monocyte TNF-alpha induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod but preserves response to R-848. J Immunol. 2004;173:4627–34. doi: 10.4049/jimmunol.173.7.4627. [DOI] [PubMed] [Google Scholar]
- 41.Netea MG, Nold-Petry CA, Nold MF, Joosten LA, Opitz B, van der Meer JH, et al. Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood. 2009 Mar 5;113(10):2324–35. doi: 10.1182/blood-2008-03-146720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009 Jul 15;183(2):787–91. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cho MH, Ahn HJ, Ha HJ, Park J, Chun JH, Kim BS, et al. Bacillus anthracis capsule activates caspase-1 and induces interleukin-1beta release from differentiated THP-1 and human monocyte-derived dendritic cells. Infect Immun. 2010 Jan;78(1):387–92. doi: 10.1128/IAI.00956-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bracci L, La Sorsa V, Belardelli F, Proietti E. Type I interferons as vaccine adjuvants against infectious diseases and cancer. Expert Rev Vaccines. 2008 Apr;7(3):373–81. doi: 10.1586/14760584.7.3.373. [DOI] [PubMed] [Google Scholar]
- 45.Levy O, Coughlin M, Cronstein BN, Roy RM, Desai A, Wessels MR. The adenosine system selectively inhibits TLR-mediated TNF-alpha production in the human newborn. J Immunol. 2006 Aug 1;177(3):1956–66. doi: 10.4049/jimmunol.177.3.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanghavi SK, Shankarappa R, Reinhart TA. Genetic analysis of Toll/Interleukin-1 Receptor (TIR) domain sequences from rhesus macaque Toll-like receptors (TLRs) 1-10 reveals high homology to human TLR/TIR sequences. Immunogenetics. 2004 Dec;56(9):667–74. doi: 10.1007/s00251-004-0734-6. [DOI] [PubMed] [Google Scholar]
- 47.Ketloy C, Engering A, Srichairatanakul U, Limsalakpetch A, Yongvanitchit K, Pichyangkul S, et al. Expression and function of Toll-like receptors on dendritic cells and other antigen presenting cells from non-human primates. Vet Immunol Immunopathol. 2008 Sep 15;125(1–2):18–30. doi: 10.1016/j.vetimm.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 48.Desrosiers MD, Cembrola KM, Fakir MJ, Stephens LA, Jama FM, Shameli A, et al. Adenosine deamination sustains dendritic cell activation in inflammation. J Immunol. 2007 Aug 1;179(3):1884–92. doi: 10.4049/jimmunol.179.3.1884. [DOI] [PubMed] [Google Scholar]
- 49.Gilles S, Fekete A, Zhang X, Beck I, Blume C, Ring J, et al. Pollen metabolome analysis reveals adenosine as a major regulator of dendritic cell-primed T(H) cell responses. J Allergy Clin Immunol. 2011 Feb;127(2):454–61. e1–9. doi: 10.1016/j.jaci.2010.12.1082. [DOI] [PubMed] [Google Scholar]
- 50.Schon MP, Schon M, Klotz KN. The Small Antitumoral Immune Response Modifier Imiquimod Interacts with Adenosine Receptor Signaling in a TLR7- and TLR8-Independent Fashion. J Invest Dermatol. 2006 Jun;126(6):1338–47. doi: 10.1038/sj.jid.5700286. [DOI] [PubMed] [Google Scholar]
- 51.Netea MG, Simon A, van de Veerdonk F, Kullberg BJ, Van der Meer JW, Joosten LA. IL-1beta processing in host defense: beyond the inflammasomes. PLoS Pathog. 2010 Feb;6(2):e1000661. doi: 10.1371/journal.ppat.1000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krumbiegel D, Zepp F, Meyer CU. Combined Toll-like receptor agonists synergistically increase production of inflammatory cytokines in human neonatal dendritic cells. Hum Immunol. 2007 Oct;68(10):813–22. doi: 10.1016/j.humimm.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 53.Lombardi V, Van Overtvelt L, Horiot S, Moingeon P. Human dendritic cells stimulated via TLR7 and/or TLR8 induce the sequential production of Il-10, IFN-gamma, and IL-17A by naive CD4+ T cells. J Immunol. 2009 Mar 15;182(6):3372–9. doi: 10.4049/jimmunol.0801969. [DOI] [PubMed] [Google Scholar]
- 54.Flach TL, Ng G, Hari A, Desrosiers MD, Zhang P, Ward SM, et al. Alum interaction with dendritic cell membrane lipids is essential for its adjuvanticity. Nat Med. 2011 Apr;17(4):479–87. doi: 10.1038/nm.2306. [DOI] [PubMed] [Google Scholar]
- 55.Burl S, Townend J, Njie-Jobe J, Cox M, Adetifa UJ, Touray E, et al. Age-dependent maturation of toll-like receptor-mediated cytokine responses in gambian infants. PLoS One. 2011;6(4):e18185. doi: 10.1371/journal.pone.0018185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wille-Reece U, Flynn BJ, Lore K, Koup RA, Miles AP, Saul A, et al. Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. J Exp Med. 2006 May 15;203(5):1249–58. doi: 10.1084/jem.20052433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adams S, O'Neill DW, Nonaka D, Hardin E, Chiriboga L, Siu K, et al. Immunization of malignant melanoma patients with full-length NY-ESO-1 protein using TLR7 agonist imiquimod as vaccine adjuvant. J Immunol. 2008 Jul 1;181(1):776–84. doi: 10.4049/jimmunol.181.1.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Du J, Wu Z, Ren S, Wei Y, Gao M, Randolph GJ, et al. TLR8 agonists stimulate newly recruited monocyte-derived cells into potent APCs that enhance HBsAg immunogenicity. Vaccine. 2010 Aug 31;28(38):6273–81. doi: 10.1016/j.vaccine.2010.06.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marodi L. Deficient interferon-gamma receptor-mediated signaling in neonatal macrophages. Acta Paediatr Suppl. 2002;91(438):117–9. doi: 10.1111/j.1651-2227.2002.tb02915.x. [DOI] [PubMed] [Google Scholar]
- 60.Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011 Feb 24;470(7335):543–7. doi: 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Demirjian A, Levy O. Safety and efficacy of neonatal vaccination. Eur J Immunol. 2008 Dec 16;39(1):36–46. doi: 10.1002/eji.200838620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lawn JE, Rudan I, Rubens C. Four million newborn deaths: is the global research agenda evidence-based? Early Hum Dev. 2008 Dec;84(12):809–14. doi: 10.1016/j.earlhumdev.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 63.WHO. The World Health Report. World Health Organization; 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.