Abstract
Objective:
The aim of this study was to determine relative contributions of transplacental flux vs. fetal production for inositol and mannose in normal term pregnancies.
Study Design:
Seven term uncomplicated pregnancies undergoing cesarean section were infused with 13C- and 2H-labeled isotopes of glucose, inositol, and mannose until a steady state was achieved. Maternal and fetal concentrations of labeled and unlabeled glucose, mannose, and inositol were measured using gas chromatography/mass spectroscopy. The fetomaternal molar percentage excess ratio was calculated for each glucose, mannose, and inositol.
Results:
The fetomaternal molar percentage excess ratio of mannose in the fetal artery (Fartery/M) was 0.99 [97.5% confidence interval (CI), 0.91–1.07] and in the fetal vein (Fvein/M), 1.02 (97.5% CI, 0.95–1.10). Both were not significantly different from 1.0, consistent with transplacental supply. The fetomaternal ratios for glucose were similar to mannose (fetal artery, 0.95; 97.5% CI, 0.84–1.15; and fetal vein, 0.96; 97.5% CI, 0.85–1.07). The fetomaternal ratio for inositol was significantly less than 1.0 (fetal artery, 0.08; 97.5% CI, 0.05–0.12; fetal vein, 0.12; 97.5% CI, 0.06–0.18), indicating little transplacental flux and significant fetal production.
Conclusion:
In normal term pregnancies, fetal mannose and glucose concentrations are dependent upon maternal transplacental supply. Fetal inositol is not dependent upon transplacental supply.
Considerable information is available about the transport of glucose in human pregnancies (1–4); little data are available for other polyols and “trace” or nonglucose carbohydrates that are present in maternal and fetal blood. The lack of data may be due to the untested assumption that other carbohydrates and polyols are produced from glucose, and fetal requirements may be met via production from maternally derived glucose (5). Based on this assumption, maternal supply of carbohydrates and polyols other than glucose would be unnecessary. Inositol can be formed through the polyol pathway (6). Mannose is biologically active as d-mannose, and inositol is biologically active as myo-inositol. Both have important roles in fetal and neonatal development.
Inositol is associated with a variety of human diseases. Some of the diseases associated with abnormal inositol metabolism are Lowe syndrome, myotublar myopathy, Charcot-Marie-Tooth type 4B1disease, Francois-Neetens fleck cornea dystrophy, and Joubert syndrome (7). Previous studies have shown relatively high concentrations of inositol from the earliest stages of human development throughout fetal life (8). Inositol and sorbitol are among the five organic osmolytes used to regulate transcellular osmotic gradients (9). Although this is well studied in renal epithelial cells, there is little information about their roles in early development within the placenta. Recently, NFAT5 (nuclear factor of activated T cells 5), a regulatory protein, has been identified as playing a role in osmolyte synthesis for inositol in the human placenta (10). Inositol has been linked to a variety of functions including its role in insulin signal transduction, intracellular calcium concentration control, and cell membrane potential (11). Inositol is also important in the formation of the neural system, and maternal inositol deficiency has been associated with the development of spina bifida in their offspring (12–14). Inositol plays a role in the regulation of surfactant phospholipid production (15, 16). It is unknown whether or not transplacental alterations of inositol could lead to abnormal function postnatally.
Recent studies on human fibroblasts have shown that the synthesis of mannose glycans requires an external supply of mannose, although theoretically it could be produced from glucose (17). Thus, the roles of inositol and mannose are important to define in human development. A recent study (18) has shown that embryonic lethality in mice with a hypomorphic phosphomannomutase 2 gene defect can be prevented by feeding the pregnant mice mannose, which indicates both the importance of mannose glycosylation during embryonic development and the availability of maternal plasma mannose to the fetuses.
In a previous study by Battaglia et al. (19), inositol levels in maternal and fetal blood for ovine pregnancies were reported. However, at that time, it was difficult to determine the concentrations of sugars and polyols present in ovine fetal blood at low concentrations due to technical limitations. As technology improved, the fetal concentrations of mannose and inositol were determined first in ovine pregnancies (20). In a subsequent clinical study by Brusati et al. (21), fetal levels of mannose were tightly correlated with maternal concentrations, which suggests dependence upon uptake from the maternal circulation. In the same study, inositol had higher fetal compared with maternal concentrations, and it was hypothesized that inositol is produced by the fetus without significant fetal transfer from the maternal circulation. Only by labeling glucose, mannose, and inositol can one definitively determine whether the fetal mannose and inositol concentrations are a result of maternal supply or derive from fetal or placental production from glucose at the fetal or placental level.
Our hypothesis was that the fetal isotopic enrichment of mannose would be approximately equal to the maternal isotopic enrichment, just as it is for glucose. Thus, without any appreciable dilution of fetal mannose, there would be no evidence of significant fetal production of mannose. For mannose, this would be further confirmed by the infusion of labeled glucosem+2 into the maternal circulation, verifying lack of fetal conversion of 13C glucosem+2 to 13C mannosem+2. These findings would establish unequivocally that fetal requirements for mannose are met primarily by transplacental transport, and not via fetal production from glucose.
Our second hypothesis is that the fetal isotopic enrichment of 2H inositolm+6 is significantly less than the maternal isotopic enrichment, demonstrating minimal transplacental flux of inositol. This would establish that fetal inositol requirements are met by fetal production from glucose rather than by transplacental transport.
Subjects and Methods
Subjects
This study was approved by the Institutional Review Boards at the two universities participating in the study. The study was supported by the National Institutes of Health, the University Pediatric Clinical Translational Research Center, the Association for Study of Malformations, and the March of Dimes. Written informed consent was obtained from each mother by one of the study investigators. Pregnant patients who were scheduled for delivery by elective cesarean section for clinical indications at term were recruited from the obstetrical services at the two participating universities. Patients undergoing elective cesarean section were chosen to eliminate the variable of active labor and labor induction agents on placental transport. The two centers collaborated on other similar projects previously.
Normal pregnancies were defined as those with mothers without chronic diseases or diseases developed during pregnancy and with ultrasound studies showing normal fetal growth rate and absence of congenital anomalies. Minorities were included as represented in the populations served at the two sites. The obstetric service at one of the university hospitals includes approximately a 30% Hispanic population and a 12% Black population. At the other university hospital, the minority representation is 70% Italian and 30% Black/Asian population. Exclusion criteria included the presence of maternal infection, chromosomal abnormalities or congenital anomalies, multiple pregnancies (twins or higher order multiple gestations), emergency cesarean sections, and mothers with chronic disease.
Study protocol
Two hours before the time of elective cesarean delivery, two maternal “arterialized” venous control samples were taken as previously described (22). The amount of infusates used is based on the maternal weight. The goal is to achieve an isotopic enrichment of between 2 and 10%. The lower limit is necessary to allow an accurate measurement of the isotopic enrichment. An upper limit is necessary to minimize altering the physiological concentrations of the compounds of interest. Each infusate underwent pyrogen and sterility testing by a research pharmacist before use. After the control samples were taken, a maternal infusion of the three carbohydrates was given. For d-[1,2-13C]glucose (glucosem+2), the infusion rate was 0.1 mg/kg · min, with a priming dose of 5.7 mg/kg as used in previous studies (20). For d-[1-13C]mannose, (mannosem+1), the infusion rate was 0.004 mg/kg · min, with a priming dose of 0.12 mg/kg. The infusion rate for [2H6]inositol (inositol m+6) was 0.004 mg/kg · min, and the priming dose was 0.05 mg/kg. The infusion of mannosem+1 and inositolm+6 into the maternal circulation over 2 h was used to establish the degree of transplacental flux of these two carbohydrates and to determine whether there is evidence of fetal and/or placental production of either of these substrates. Before delivery, a minimum of two samples were taken 30 min apart to determine whether (verify that) a steady state was accomplished. At the time of delivery, a third maternal arterialized venous sample was taken along with arterial and venous umbilical cord blood samples. The glucosem+2 infusion was given to determine the conversion of glucose to mannosem+2.
Carbohydrate analysis
Allose and chiro-inositol, which are stereoisomers of mannose and inositol, respectively, were used as internal standards to account for the loss of carbohydrate during the following sample preparation and derivatization steps. The internal standards have different retention times from the compounds of interest on the gas chromatographic system. Thus, the order of elution is allose, mannose, glucose, chiro-inositol, and myo-inositol. There is baseline separation of all these compounds using this chromatographic system. Human plasma glucose concentration is approximately 40-fold higher than mannose concentration. Chromatographically, the high glucose concentration interferes with the determination of mannose concentration. Therefore, for the determination of mannose enrichment, glucose was removed from each sample by the action of glucose oxidase (23). Plasma (0.05 ml) was added to 0.2 ml water and 0.05 ml of internal standards (10 mg/liter of chiro-inositol and 10 mg/liter of allose), followed by 280 U of catalase and 12 U of glucose oxidase. After incubating at ambient temperatures for 90 min, the reaction was stopped by the addition of 0.15 ml of 0.3 mol/liter ZnSO4 and 0.15 ml of 0.3 mol/liter Ba(OH)2. The protein precipitate was removed by centrifugation at 13,000 × g for 4 min at 4 C. The supernatant was transferred to a test tube and dried under a vacuum. For glucose enrichment, only 0.01 ml plasma was used, and the glucose oxidase treatment was omitted.
Isotopic enrichments were determined using gas chromatography/mass spectroscopy (model 5975; Agilent Technologies, Santa Clara, CA) equipped with an HP5-MS column (30 m × 0.25 mm × 0.25 μm). Helium was used as the carrier gas at a flow rate of 1 ml/min. The injector inlet and the transfer line were both maintained at 280 C. The initial column temperature was 120 C and held for 1 min. The column temperature was then ramped at a rate of 12 C/min to 179 C and held for 8 min. Finally, column temperature was ramped at a rate of 50 C/min to 320 C and held for 1 min to clean the column. Mannose was converted to the aldononitrile peracetate, and inositol was converted to the peracetate (21). A total of 0.1 ml hydroxylamine hydrochloride (20 g/liter in anhydrous pyridine) was added to the dried residue. The pyridine solution was incubated at 90 C for 30 min. After cooling, 0.075 ml of acetic anhydride was added, and the solution was incubated for another 30 min at 90 C. After cooling, 1 ml of 1 mol/liter HCl was added, followed by 2 ml chloroform. The mixture was vortexed, and after the separation of the phases, the aqueous layer was removed and discarded. The chloroform solution was washed with another 1 ml of 1 mol/liter HCl, then washed sequentially three times using 1 ml of water and then dried. The residue was dissolved in 0.05 ml acetonitrile. Mannose was monitored at a mass/charge ratio of 314, 315, 316, 319, 320, and 321. Inositol was monitored at a mass/charge ratio of 373, 374, 375, 379, 380, and 381. For mannose, the ratio of the 315:314 peaks was used to monitor the m+1 molar percentage excess (MPE), and the ratio of the 316:314 peaks was used to monitor the m+2 MPE. For inositol, the ratio of the 375:373 peaks was used to monitor the m+2 MPE, and the ratio of the 379:373 peaks was used to monitor the m+6 MPE. The plasma concentrations of mannose and inositol were calculated using the total ion counts of the corresponding ions. The total ion counts were compared with standard curves constructed using unlabeled mannose and inositol.
Calculations and statistics
Maternal and fetal characteristics are calculated as means, ranges, number and percentage. The mean maternal MPE (Mmpe) is calculated as the mean of the Mmpe measurements once a steady state was achieved. The mannose and inositol ratios of fetal MPE (Fmpe)/Mmpe are calculated with 97.5% confidence intervals (CI) to demonstrate equivalence to, or difference from, 1. A secondary analysis tests that the slope in a linear regression of Fmpe as a function of Mmpe is equal to 1.
Results
From both university hospital study sites, a total of seven study subjects completed the study protocol. A total of five mother-fetus pairs were enrolled at one university hospital, and two patients were enrolled at the other university hospital. There were no patients enrolled who did not complete the study, and there were no observed complications from the infusate. Characteristics of the studied population are presented in Table 1. All newborns were appropriate for gestational age for birth weight; oxygenation and acid-base balance were within the normal range for all of the studied fetuses according to data published previously (23).
Table 1.
Maternal, fetal, and newborn infant clinical data
| Variable | |
|---|---|
| Maternal age (yr) | 31.1 (27–41) |
| Race | |
| White | 5 (71%) |
| Black | 1 (14%) |
| Hispanic | 1 (14%) |
| Multiparity (no. of patients) | 3 |
| Gestational age at delivery (wk) | 39.1 (38.8–39.5) |
| Cesarean delivery indication | |
| Repeat | 3 (43%) |
| Breech | 4 (57%) |
| Sex | |
| Male | 5 (71%) |
| Female | 2 (29%) |
| Birthweight (g) | 3369 (3180–3632) |
Data are expressed as mean (range) or number (percentage).
Carbohydrate enrichments
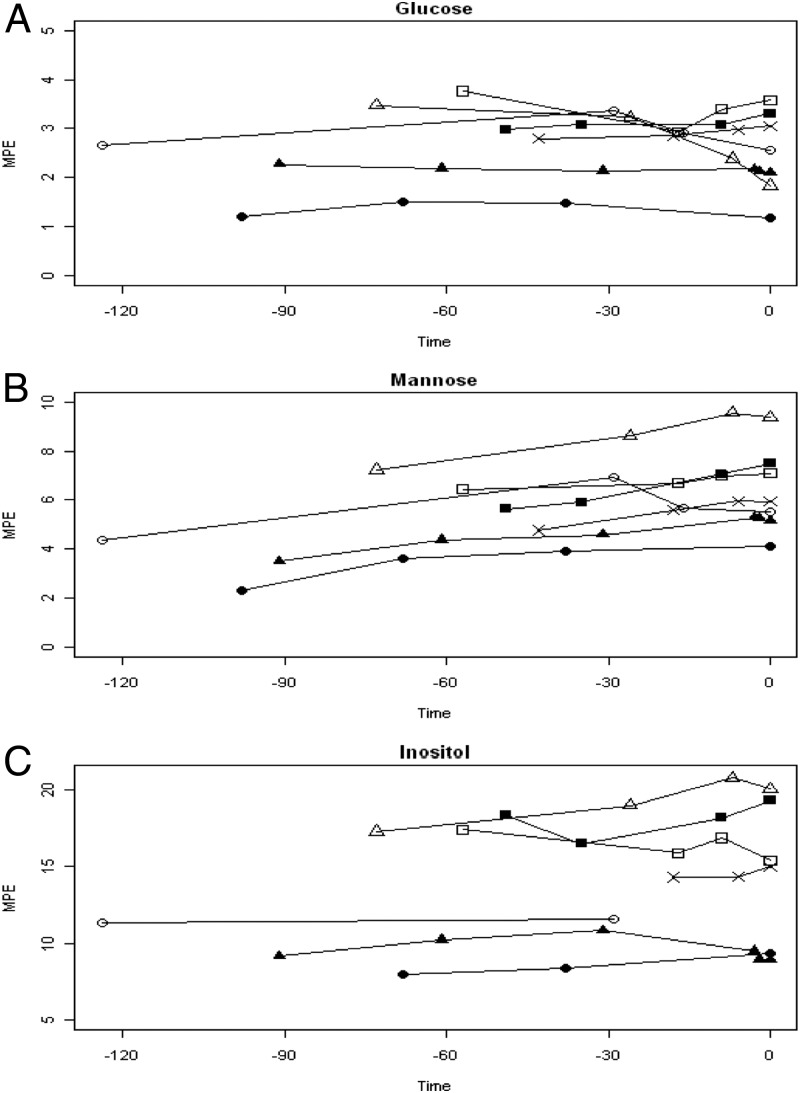
The maternal plasma MPE after bolus infusion for glucose, mannose, and inositol is depicted in Fig. 1, A–C. Serial maternal samples were taken before delivery to ensure that the infusate was at a relative steady state before delivery.
Fig. 1.
Maternal plasma MPE for glucose (A), mannose (B), and inositol (C) after bolus of infusate, with time 0 being delivery and with −30, −60, −90, and −120 indicating minutes before delivery. There are seven lines, with each line/symbol representing a maternal subject and each symbol indicating a maternal sample.
The fetal arterial and venous plasma enrichments for glucose (m+2), mannose (m+1), and inositol (m+6) are presented in Table 2. There were no significant amounts of m+1 or m+6 detected for glucose, with the primary form being m+2. For mannose, there were no significant amounts of m+2 or m+6 detected, with the primary form being m+1. For inositol, there were no significant amounts of m+1 or m+2 detected, with the primary form being m+6. (The significance of this is that m+2 enrichment for mannose and inositol was negligible.)
Table 2.
Plasma enrichments for glucose (m+2), mannose (m+1), and inositol (m+6)
| Patient no. | Glucose (m+2) |
Mannose (m+1) |
Inositol (m+6) |
|||
|---|---|---|---|---|---|---|
| Arterial | Venous | Arterial | Venous | Arterial | Venous | |
| 1 | 3.26 | 3.30 | 6.43 | 6.46 | 1.32 | 1.31 |
| 2 | 2.89 | 2.82 | 5.94 | 5.91 | 1.53 | 2.22 |
| 3 | 2.11 | 1.96 | 7.76 | 8.28 | 1.05 | 1.67 |
| 4 | 2.60 | 2.95 | 5.47 | 5.88 | 1.31 | 1.92 |
| 5 | 3.30 | 3.40 | 6.80 | 7.39 | 1.83 | 2.48 |
| 6 | 1.16 | 1.18 | 3.74 | 3.65 | 0.46 | 0.41 |
| 7 | 2.10 | 2.08 | 4.32 | 4.57 | 0.75 | 1.58 |
| Mean (sd) | 2.49 (0.76) | 2.53 (0.81) | 5.78 (1.40) | 6.02 (1.58) | 1.18 (0.47) | 1.66 (0.68) |
Data are expressed as fetal arterial and venous MPE.
Fetal-to-maternal enrichment ratios
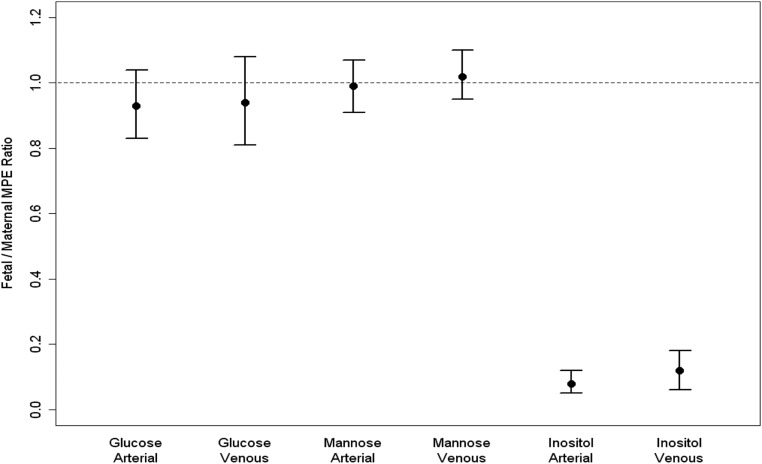
Figure 2 presents the fetal to maternal (F/M) MPE ratios for both the umbilical vein and umbilical artery for glucose, mannose, and inositol. The Fartery/M MPE and the Fvein /M MPE for mannose was 0.99 (97.5% CI, 0.91–1.07) and 1.02 (97.5% CI, 0.95–1.10), respectively. We consider these estimates to be equivalent to 1 because the 97.5% CI do not include estimates that would be considered clinically different from 1. The Fartery/M MPE and the Fvein/M MPE for inositol was 0.08 (97.5% CI, 0.05–0.12) and 0.12 (97.5% CI, 0.06–0.18), respectively. For mannose and glucose, the slope estimates from linear regressions of the Fmpe as a function of the Mmpe were not significantly different from 1, with estimates of 0.91 (97.5% CI, 0.56–1.27) and 1.08 (97.5% CI, 0.43–1.73), respectively. For inositol, the slope estimate is significantly different from 1, with an estimate of 0.08 (97.5% CI, −0.12 to 0.28; P < 0.0001).
Fig. 2.
Fetomaternal MPE ratios for glucose, mannose, and inositol and 97.5% CI.
Discussion
There have been several studies describing transplacental gradients of glucose in both animal and human subjects (1–4, 20, 21). Our study presents the first data describing the normal steady-state fetal-to-maternal plasma enrichments for mannose and inositol using stable isotopes. Previous studies have described the maternal and fetal concentrations of mannose and inositol in pregnant sheep and also in humans. These studies established that there were significant umbilical veno-arterial concentration differences for glucose and mannose in human and ovine pregnancies. However, there was no measurable umbilical uptake of inositol in human pregnancies. Because stable isotopes were not used, these studies could not determine the origin of fetal inositol and fetal mannose. The present study extends those observations by examining the fetal/maternal plasma enrichment ratios for the three carbohydrates after a steady-state infusion into the maternal circulation. In both the Teng et al. (20) and Brusati et al. (21) studies, there was a higher level of inositol present in the fetus than in the maternal circulation, indicating either active transport against a concentration gradient or fetal-placental production of inositol.
The F/M MPE ratios of 0.99 for mannose and 0.08 for inositol support our hypothesis. More specifically, the F/M MPE ratio of 0.99 clearly demonstrates that over 95% of fetal plasma mannose originates from transplacental maternal supply and follows its concentration gradient from mother to fetus, similar to that of glucose. The F/M MPE ratio for glucose was 0.93, which is similar to prior studies (1–4) and, once again, confirms that in normal human pregnancies, there is little if any fetal gluconeogenesis taking place. In contrast, the higher fetal concentration of inositol does not appear to be maternally derived with an F/M MPE ratio of 0.09, which establishes that less than 10% of fetal plasma inositol is derived from maternal plasma. This observation in term pregnancies is especially interesting in light of a prior study (8) showing that inositol levels are elevated during the first trimester. Our study in term pregnancies shows that little of the fetal plasma inositol is maternally derived, but it raises questions of why inositol levels in the first trimester are elevated and whether the fetal inositol is of maternal origin. One theory for this apparent discrepancy is that the fetus may be more dependent on maternal inositol transfer in the first trimester of pregnancy than at full term.
Labeled glucose was used to confirm that the study design would yield glucose results comparable to previous studies and, secondarily, to determine whether the fetal mannose was produced from maternally derived glucose. In our study, there was no detectable conversion of 13C glucosem+2 to 13C inositolm+2 or 13C mannosem+2. For mannose, these results are significant and support the conclusion that fetal plasma mannose is derived from maternal plasma mannose by transplacental passage. However, the absence of glucose labeling of inositol carries little biological significance because inositol concentrations in placental tissue and fetal tissues are very high. Given a large intracellular pool, a much longer duration of maternal infusion would be required to detect any significant enrichment of 13C inositolm+2 from 13C glucosem+2.
A limitation of our study is that although the maternal concentrations appear to be at a steady state at the time of umbilical sampling, the in utero metabolism and turnover for inositol and mannose are unknown, and the 2-h infusion before sampling may have been inadequate for inositol. The study was limited to one fetal sample obtained at delivery via the umbilical artery and vein cord segment. Ideally, uterine and umbilical arteriovenous differences could be continuously monitored before delivery and over a longer time, which is a major limitation for human studies but is eminently achievable in animal studies. The fetal turnover rates or plasma rate of appearance (Ra) for inositol and mannose are unknown. Recently, the Ra for inositol and mannose was described by Brown et al. (24) and demonstrated a relatively high requirement for de novo production from glucose. Without knowledge of fetal inositol Ra, we must use caution when interpreting the results. Our results suggest that the lack of labeled inositol in the fetus is because inositol is not primarily maternally derived; however, it is also possible that if the Ra is slow, the duration of inositol infusion may not be sufficient to capture the origin of inositol. More information regarding the fetal Ra would be needed to definitively conclude that fetal inositol is not maternally derived.
The finding in our study, along with other normal human and sheep pregnancy studies, of a significant transplacental flux of mannose into the fetal circulation suggests the presence of a placental transporter (20, 21). Future research efforts are needed to identify a specific placental high-affinity transporter for mannose that has been identified in other tissues (25). Whether the transplacental supply of mannose and fetal production of inositol remains unaltered in aberrancies of fetal growth (intrauterine growth restriction and macrosomia) or with maternal diabetes remains unknown.
Acknowledgments
This work was supported by National Institutes of Health Grant R01 HD34837-05A2, March of Dimes Grant FY00-738, and Pediatric Clinical Translational Research Center/National Institutes of Health Grant MO1 RR00069, and by the Association for Study of Malformations.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CI
- Confidence interval
- F
- fetal
- M
- maternal
- MPE
- molar percentage excess
- Ra
- rate of appearance.
References
- 1. Stembera ZK, Hodr J. 1966. The relationship between the blood levels of glucose, lactic acid and pyruvic acid in the mother and in both umbilical vessels of the healthy fetus. Biol Neonat 10:227–238 [DOI] [PubMed] [Google Scholar]
- 2. Bozzetti P, Ferrari MM, Marconi AM, Ferrazzi E, Pardi G, Makowski EL, Battaglia FC. 1988. The relationship of maternal and fetal glucose concentrations in the human from midgestation until term. Metabolism 37:358–363 [DOI] [PubMed] [Google Scholar]
- 3. Nicolini U, Hubinont C, Santolaya J, Fisk NM, Coe AM, Rodeck CH. 1989. Maternal fetal glucose gradient in normal pregnancies and in pregnancies complicated by alloimmunization and fetal growth retardation. Am J Obstet Gynecol 161:924–927 [DOI] [PubMed] [Google Scholar]
- 4. Marconi AM, Paolini C, Buscaglia M, Zerbe G, Battaglia FC, Pardi G. 1996. The impact of gestational age and fetal growth on the maternal-fetal glucose concentration difference. Obstet Gynecol 87:937–942 [DOI] [PubMed] [Google Scholar]
- 5. Berrone E, Beltramo E, Solimine C, Ape AU, Porta M. 2006. Regulation of intracellular glucose and polyol pathway by thiamine and benfotiamine in vascular cells cultured in high glucose. J Biol Chem 281:9307–9313 [DOI] [PubMed] [Google Scholar]
- 6. Kalhan SC. 2009. Nonglucose carbohydrates and infant nutrition and metabolism. J Nutr 139:1611–1612 [DOI] [PubMed] [Google Scholar]
- 7. Berry GT. 2011. Is prenatal myo-inositol deficiency a mechanism of CNS injury in galactosemia. J Inherit Metab Dis 34:345–355 [DOI] [PubMed] [Google Scholar]
- 8. Jauniaux E, Hempstock J, Teng C, Battaglia FC, Burton GJ. 2005. Polyol concentrations in the fluid compartments of the human conceptus during the first trimester of pregnancy: maintenance of redox potential in a low oxygen environment. J Clin Endocrinol Metab 90:1171–1175 [DOI] [PubMed] [Google Scholar]
- 9. Burg MB. 1995. Molecular basis of osmotic regulation. Am J Physiol 268:F983–F996 [DOI] [PubMed] [Google Scholar]
- 10. Arroyo JA, Teng C, Battaglia FC, Galan HL. 2009. Determination of the NFAT5/TonEBP transcription factor in the human and ovine placenta. Syst Biol Reprod Med 55:164–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ooms LM, Horan KA, Rahman P, Seaton G, Gurung R, Kethesparan DS, Mitchell CA. 2009. The role of the inositol polyphosphate 5-phosphatases in cellular function and human disease. Biochem J 419:29–49 [DOI] [PubMed] [Google Scholar]
- 12. Greene ND, Copp AJ. 2005. Mouse models of neural tube defects: investigating preventive mechanisms. Am J Med Genet C Semin Med Genet 135C:31–41 [DOI] [PubMed] [Google Scholar]
- 13. Groenen PM, Peer PG, Wevers RA, Swinkels DW, Franke B, Mariman EC, Steegers-Theunissen RP. 2003. Maternal myo-inositol, glucose, and zinc status is associated with the risk of offspring with spina bifida. Am J Obstet Gynecol 189:1713–1719 [DOI] [PubMed] [Google Scholar]
- 14. Reece EA, Khandelwal M, Wu YK, Borenstein M. 1997. Dietary intake of myo-inositol and neural tube defects in offspring of diabetic rats. Am J Obstet Gynecol 176:536–539 [DOI] [PubMed] [Google Scholar]
- 15. Hallman M, Saugstad OD, Porreco RP, Epstein BL, Gluck L. 1985. Role of myoinositol in regulation of surfactant phospholipids in the newborn. Early Hum Dev 10:245–254 [DOI] [PubMed] [Google Scholar]
- 16. Hallman M, Bry K, Hoppu K, Lappi M, Pohjavuori M. 1992. Inositol supplementation in premature infants with respiratory distress syndrome. N Engl J Med 326:1233–1239 [DOI] [PubMed] [Google Scholar]
- 17. Panneerselvam K, Etchison JR, Freeze HH. 1997. Human fibroblasts prefer mannose over glucose as a source of mannose for N-glycosylation. Evidence for the functional importance of transported mannose. J Biol Chem 272:23123–23129 [DOI] [PubMed] [Google Scholar]
- 18. Schneider A, Thiel C, Rindermann J, DeRossi C, Popovici D, Hoffmann GF, Gröne HJ, Körner C. 2011. Successful prenatal mannose treatment for congenital disorder of glycosylation-Ia in mice. Nat Med 18:71–73 [DOI] [PubMed] [Google Scholar]
- 19. Battaglia FC, Meschia G, Blechner JN, Barron DH. 1961. The free myoinositol concentration of adult and fetal tissues of several species. Q J Exp Physiol Cogn Med Sci 46:188–193 [DOI] [PubMed] [Google Scholar]
- 20. Teng CC, Tjoa S, Fennessey PV, Wilkening RB, Battaglia FC. 2002. Transplacental carbohydrate and sugar alcohol concentrations and their uptakes in ovine pregnancy. Exp Biol Med (Maywood) 227:189–195 [DOI] [PubMed] [Google Scholar]
- 21. Brusati V, Józwik M, Józwik M, Teng C, Paolini C, Marconi AM, Battaglia FC. 2005. Fetal and maternal non-glucose carbohydrates and polyols concentrations in normal human pregnancies at term. Pediatr Res 58:700–704 [DOI] [PubMed] [Google Scholar]
- 22. Sonnenberg GE, Keller U. 1982. Sampling of arterialized heated-hand venous blood as a noninvasive technique for the study of ketone body kinetics in man. Metabolism 31:1–5 [PubMed] [Google Scholar]
- 23. Battaglia FC, Meschia G. 1986. An introduction to fetal physiology. Orlando, FL: Academic Press: 70–86 [Google Scholar]
- 24. Brown LD, Cheung A, Harwood JE, Battaglia FC. 2009. Inositol and mannose utilization rates in term and late pre-term infants exceed nutritional intakes. J Nutr 139:1648–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Panneerselvam K, Freeze HH. 1996. Mannose enters mammalian cells using a specific transporter that is insensitive to glucose. J BiolChem 271:9417–9421 [DOI] [PubMed] [Google Scholar]