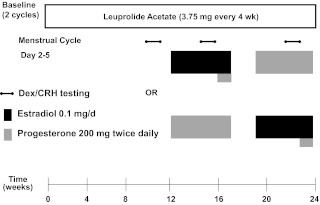
Fig. 1.
Study schematic and Dex/CRH testing. All participants received injections of the GnRH agonist leuprolide acetate (Lupron) at a dose of 3.75 mg every 4 wk. Plasma FSH, LH, estradiol, and progesterone levels were checked at each study visit to confirm adequate gonadal suppression. After 3 months of unopposed leuprolide acetate, all participants entered the hormonal addback phase while continuing to receive monthly leuprolide injections. The women were randomly assigned to receive 5 wk each of transdermal 17β-estradiol (E) (Watson Pharmaceuticals, Inc., Corona, CA; Novum Pharmaceutical Research Services, Pittsburgh, PA; Novartis Pharmaceuticals Corp., East Hanover, NJ) at a dose of 0.1 mg/d and progesterone (P) vaginal suppositories (Upsher-Smith Laboratories, Minneapolis, MN; NIH Pharmacy, Bethesda, MD) at a dose of 200 mg twice daily in a double-blind, placebo-controlled, crossover fashion with a 2-wk washout period between the periods of hormone administration. In addition, during the fifth week of estradiol, all women received both estradiol and progesterone to induce menses. During the hormonal addback phases, all women received both active or placebo patches and suppositories each day to blind both researchers and participants to the hormonal addback. Dex/CRH testing was developed for affective disorders by Holsboer and colleagues (37). Participants take 1.5 mg Dex orally at 2300 h the night before the test. The next day, while fasting, participants present to the outpatient clinic to have blood sampling while resting in a supine position. Baseline samples are drawn at 1445 and 1455 h. At 1500 h, participants are given ovine CRH 100 μg iv, and blood is drawn at 15, 30, 45, 60, and 75 min after the CRH infusion.