Abstract
Context:
Menstruation is preceded by progesterone withdrawal and endometrial matrix remodeling predominantly through induction of matrix metalloproteinases (MMP) and recruitment of invading neutrophils.
Design:
Using endometrial tissues from women during various phases of the menstrual cycle, we found that MMP2, MMP9, and MMP11 were up-regulated in the late secretory phase/premenstrual phase. Because TGFβ-responsive genes were also up-regulated in endometrium during this time, we tested the hypothesis that TGFβ1 and progesterone regulate expression of MMP in human endometrial stromal cells (HESC).
Results:
Treatment of HESC with TGFβ1 resulted in marked increases in MMP2 and MMP11 mRNA and pro- and active MMP2 activity. Progesterone inhibited TGFβ1-induced stimulation of MMP2 and MMP11 through its nuclear hormone receptors. Interestingly, TGFβ1 also decreased progesterone receptor (PR)-A and PR-B in HESC with a more pronounced effect on PR-A.
Conclusions:
These data support the hypothesis that TGFβ1 has endogenous anti-progestational effects in HESC and that the opposing effects of progesterone and TGFβ1 are important in regulation of matrix integrity in human endometrium.
Normal menstruation is orchestrated through hormone-hormone receptor interactions and through locally produced paracrine factors that mediate cell-cell and cell-matrix communications (1–4). In women, expression of progesterone receptors (PR) in the endometrium varies throughout the menstrual cycle. In the presence of a blastocyst, the endometrial stromal cell decidualizes, loses expression of the PR-B isoform, and facilitates implantation. In the absence of implantation, stromal cells respond to withdrawal of progesterone and initiate menstruation (5) characterized by up-regulation of proteolytic enzymes. Microarray studies indicate that several proteases are up-regulated in late secretory endometrium including matrix metalloproteinase 2 (MMP2) and MMP11 (6). MMP are zinc-containing endopeptidases that enzymatically digest certain extracellular matrix (ECM) proteins and therefore play important roles in tissue-remodeling processes (7, 8) Endometrial expression of MMP is highly regulated during the menstrual cycle to facilitate successful implantation or to accomplish timely degradation and remodeling of the endometrial lining during menstruation (9, 10).
During the late luteal phase (a time in which progesterone levels decrease substantially), up-regulation of proteases coincides with increased expression of several TGFβ-responsive genes. TGFβ1, a secreted homodimeric protein, is the prototypic member of a family of approximately 40 structurally related proteins known as the TGFβ superfamily. In several progesterone-responsive cells, TGFβ1 compromises expression of PR (5) and progesterone action by inhibiting PR-mediated gene transcription leading to induction of inflammatory response pathways and activation of nuclear factor-κB, a transcription factor that further antagonizes PR function (11–13). Examples of these effects of TGFβ1 include inhibition of progesterone to modify mRNA levels of connexin43 (Cx43), PTHrP, endothelin (ET-l), and enkephalinase (14–17). Thus, several lines of evidence suggest that TGFβ1 opposes the effect of progesterone in progesterone-responsive tissues. Here, we tested the hypothesis that TGFβ1 and progesterone regulate expression of MMP in human endometrial stromal cells (HESC).
Materials and Methods
Endometrial tissue
Human endometrial tissues were obtained from hysterectomy specimens performed due to benign nonendometrial pathology (e.g. premalignant disease of the cervix, uterine prolapse, and leiomyomas) under a protocol approved by the Institutional Review Board at the University of Texas Southwestern Medical Center. All patients were parous, 26–51 yr of age, menstruating regularly, and free of any hormone treatment for more than 30 d before surgery. Histopathological examination of the formalin-fixed portion of the endometrium excluded pathology and identified the corresponding day of the menstrual cycle (18). Tissues were classified as proliferative (d 5–13), early secretory (d 15–21), and late secretory (d 22–26). Tissues from d 28 with evidence of hemosiderin or erythrocytes in the stroma were classified as premenstrual.
ESC isolation procedure and cell culture conditions
ESC were isolated from specimens in the secretory phase unless stated otherwise. Specifically, normal ESC were separated from epithelial glands by digesting the tissue fragments with collagenase, as previously described (19). Briefly, tissue was minced into 2- to 3-mm pieces and incubated with collagenase (1 mg/ml) and deoxyribonuclease (0.15 mg/ml) in Hank's balanced solution with stirring for 30 min at 37 C. The suspension was filtered through a 150-μm wire sieve to remove mucus and undigested tissue. The filtrate was then passed through a 70-μm wire sieve, which allowed the stromal cells to pass through while intact glands were retained. Glands were backwashed, plated at high density, and used for experiments within 7 d. Pelleted ESC were washed three times with serum-free DMEM (Life Technologies, Inc.-BRL, Rockville, MD), plated at 5 × 105 cells/cm2 in DMEM supplemented with 10% heat-inactivated fetal bovine serum (Life Technologies, Inc.-BRL), streptomycin (100 U/ml) (Life Technologies, Inc.- BRL), and penicillin (100 U/ml) (Life Technologies, Inc.-BRL). Culture medium was replaced every 3 d. After one passage (P1) using standard trypsinization methods, cells were more than 99% pure as analyzed by immunocytochemical staining with antibodies to vimentin (V9; Dako, Copenhagen, Denmark), cytokeratin (Dako), factor VIII (Dako), and leukocyte common antigen (2B11 plus PD7/26; Dako), and were then used for experiments, unless noted as high passage (P7). Cells isolated from each individual patient were used for one experiment at a time. Each experiment was performed in triplicate and repeated in at least three cell preps.
TGFβ1 and progesterone treatment
TGFβ1 and progesterone treatment was conducted in six-well plates. Preconfluent ESC (passage 1) were placed in serum-free medium for 24 h at 37 C before treatment. Thereafter, cells were treated with serum-free medium containing vehicle, various concentrations of TGFβ1 (0.1–5 ng/ml), progesterone (0.1–100 nm), or both. Medium was collected 48 h after treatment and stored at −80 C until assayed. Experiments were performed in triplicate plates.
Gelatin zymography
MMP2 and MMP9 activity in the culture media was analyzed by zymography. Quantitative gelatin zymography was performed as described (20). Serum-free media were concentrated by centrifugal filter (Amicon, Billerica, MA), protein concentrations were determined using a bicinchoninic acid protein assay (Pierce, Rockford, IL), and samples (4 μg) were applied in each lane.
RNA extraction from endometrial tissue and quantitative real-time PCR
Total cellular RNA was extracted from frozen endometrial tissue or cultured HESC using RNAqueous-4PCR Kit (Ambion, Austin, TX), according to the manufacturer's protocol. Reverse transcription reactions were conducted with 1 μg total RNA in a reaction volume of 20 μl. Each reaction contained 10 mm dithiothreitol, 0.5 mm deoxynucleotide triphosphates, 0.015 μg/μl random primers, 40 U ribonuclease inhibitor (Invitrogen, Carlsbad, CA; catalog item 10777-019), and 200 U reverse transcriptase (Invitrogen; catalog item 18064-014). Reaction conditions were 10 min at 23 C and 60 min at 50 C followed by 70 C for 15 min. For real-time PCR, primer sequences to amplify MMP2 were 5′-TTGATGGCATCGCTCAGATC-3′ (sense) and 5′-TGTCACGTGGCGTCACAGT-3′ (antisense); MMP9, 5′-CCACCACAACATCACCTATTGG-3′ (sense) and 5′-GCAAAGGCGTCGTCAATCA-3′ (antisense); MMP11, 5′-CTTCCGAGGCAGGGACTACT-3′ (sense) and 5′-AGAAGTCAGGACCCACGAGA-3′ (antisense); INHβA, 5′-CCCTTTAAGCCCACTTCCTC-3′ (sense) and 5′-CCTCGGAGATCATCACGTTT-3′ (antisense); TGFβ1, 5′-AAATTGAGGGCTTTCGCCTTA-3′ (sense) and 5′-GAACCCGTTGATGTCCACTTG-3′ (antisense); TGFβ2, 5′-GCACAACCGGCGGAAGA-3′ (sense) and 5′-CAGCAATTATCCTGCACATTTCTAA-3′ (antisense); TGFβ3, 5′-CCTTTCAGCCCAATGGAGATAT-3′ (sense) and 5′-CATTGTCCACGCCTTTGAATT-3′ (antisense); PR-B, 5′-CGGACACCTTGCCTGAAGTT-3′ (sense) and 5′-CAGGGCCGAGGGAAGAGTAG-3′ (antisense); total PR (C terminus), 5′-AATCATTGCCAGGTTTTCGAA-3′ (sense) and 5′-GCCCACTGACATGTTTGTAGGA-3′ (antisense); and GAPDH, 5′-GGAGTCAACGGATTTGGTCGTA-3′ (sense) and 5′-CAACAATATCCACTTTACCAGAGTTA-3′ (antisense). Primers were chosen so that the resulting amplicons would cross an exon junction, thereby eliminating false-positive signals from genomic DNA contamination. Gene expression was normalized to expression of GAPDH. Primer sets were tested to ensure that efficiency of amplification over a wide range of template concentrations was equivalent to that of GAPDH. PCR was carried out in the ABI 7900HT sequence detection system (Applied Biosystems, Foster City, CA). The reverse transcription product from 25 ng RNA was used as template. Reaction volumes were 15 μl, containing 1× Master Mix (Applied Biosystems; catalog item 4309155 for SYBR Green). Primer concentrations were 900 nm. Cycling conditions were 2 min at 50 C, followed by 10 min at 95 C and then 40 cycles of 15 sec at 95 C and 1 min at 60 C. A preprogrammed dissociation protocol was used after amplification to ensure that all samples exhibited a single amplicon. Levels of mRNA were determined using the delta-delta cycle threshold method (Applied Biosystems).
Immunoblotting analysis
HESC were plated in 10-cm dishes and cultured until preconfluent. Preconfluent cultures were placed in serum-free medium for 24 h before treatment. The optimal time for stimulation was determined by a time-course study performed as background experiments. After treatment, cells were washed with PBS, and whole-cell extracts were prepared by lysing the cells in lysis buffer (50 mm Tris-HCl, 125 mm NaCl, 0.1% Nonidet P-40, 5 mm EDTA, 50 mm NaF, and 0.1% phenylmethylsulfonyl fluoride). The lysate was centrifuged at 15,000 × g for 15 min at 4 C, and supernatant was collected. Total protein concentrations were quantified using the bicinchoninic acid protein assay reagent (Pierce). Whole-cell protein extract was resolved with SDS-PAGE using a 10% polyacrylamide gel under reduced conditions. After transfer to a nitrocellulose membrane (Bio-Rad, Hercules, CA), the protein was stained with Ponceau S (Aldrich, Milwaukee, WI) to verify uniform loading and transfer. The membranes were blocked with 5% powdered skim milk (Bio-Rad) in Tris-buffered saline with Tween 20 (TBST) [50 mm Tris-HCl, 150 mm NaCl, 0.1% Tween 20 (pH 7.4)] overnight and subsequently incubated with primary antibodies [PR (Clone SP2; Thermo Scientific, Barrington, IL); and GAPDH (Ambion)] for 1 h at room temperature. The membrane was then washed three times with TBST and incubated with the appropriate horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. Subsequently, the membrane was washed three times with TBST and analyzed by enhanced chemiluminescence (GE Healthcare, Little Chalfont, UK).
Ectopic overexpression of PR
For overexpression of PR, advanced-passage cervical stromal cells were infected with either control adenovirus or PR-A- or PR-B-expressing adenovirus (108 plaque-forming units) for 24 h (21). Thereafter, cells were treated with vehicle, TGFβ1, progesterone, or TGFβ1 plus progesterone as indicated.
Results
MMP and TGFβ gene expression in endometrial tissues
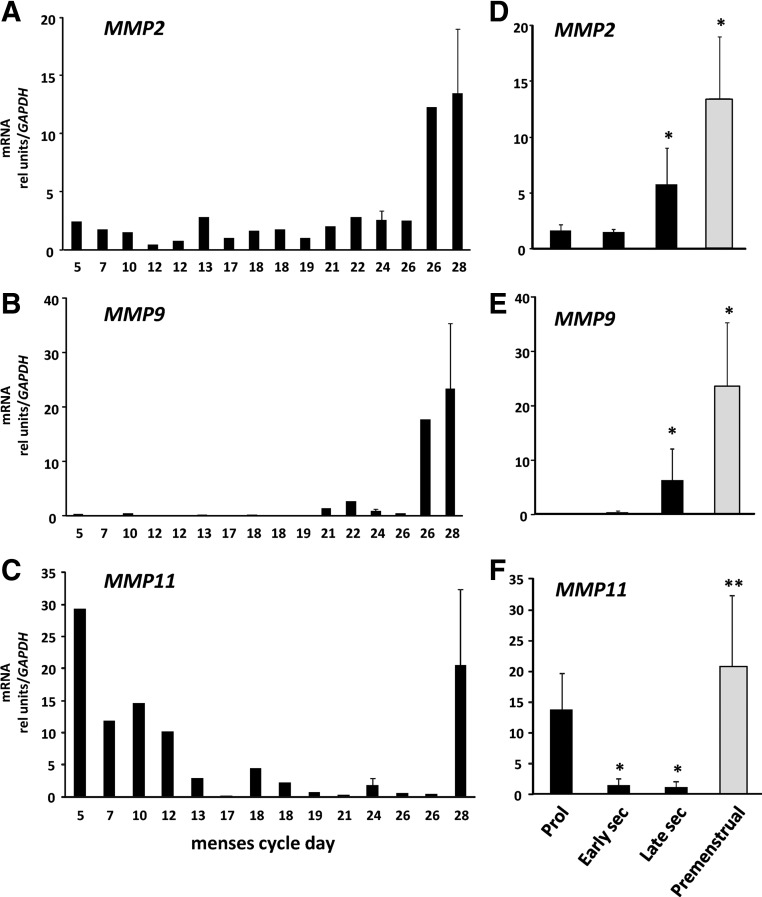
MMP2 and MMP9 are believed to play important roles in tissue remodeling in a number of tissues. To determine whether gene expression for these two proteases was regulated in endometrial tissues throughout the menstrual cycle, MMP2 and MMP9 mRNA levels were quantified in endometrial tissues from ovulatory women in various stages of the cycle (Fig. 1). MMP2 and MMP9 mRNA were regulated in human endometrial tissues during the menstrual cycle. Specifically, mRNA levels of both proteases were increased significantly in endometrial tissues from the late secretory and premenstrual phases with rapid resolution in the proliferative phase. MMP11 gene expression was also increased in premenstrual tissues (12- to 50-fold). In contrast with MMP2 and MMP9, however, MMP11 mRNA remained increased during the proliferative phase falling to baseline levels after midcycle (Fig. 1).
Fig. 1.
Regulation of MMP2 and MMP9 mRNA in human endometrium. Levels of MMP2 (A), MMP9 (B), and MMP11 (C) mRNA were quantified using quantitative PCR and expressed relative to that of GAPDH as a function of menstrual cycle day. Error bars indicate three to four tissues per day. Data in D–F represent mean ± sem of tissues obtained in proliferative (Prol) (n = 6), early secretory (sec) (n = 5), late secretory (n = 5), and premenstrual (n = 4) phase. *, P < 0.05 compared with proliferative; **, P < 0.05 compared with early or late secretory, ANOVA.
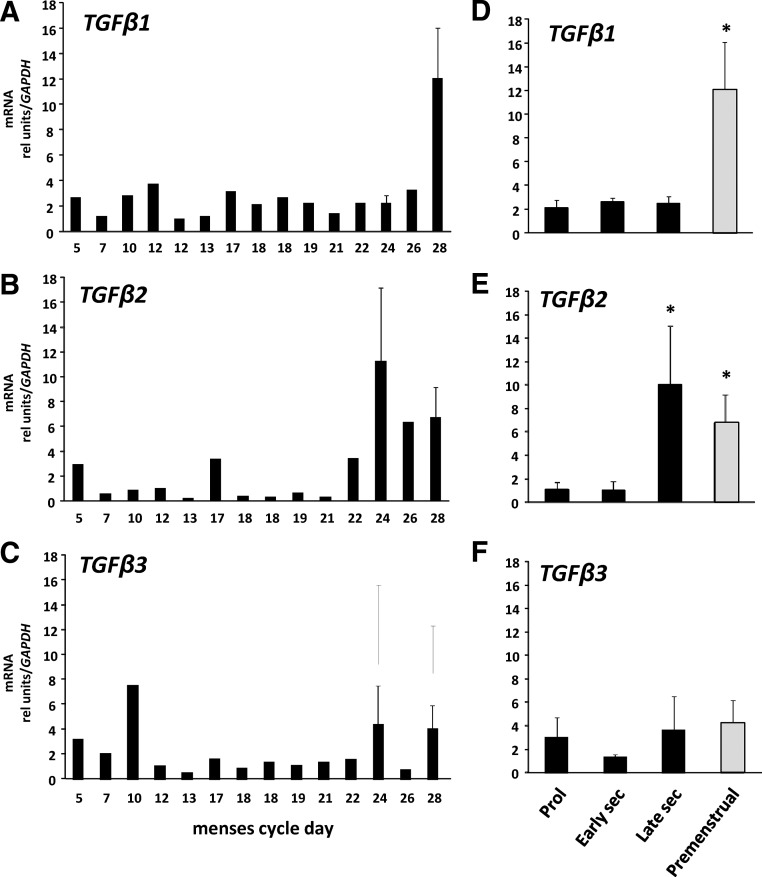
Previous studies have shown that TGFβ-related genes, including inhibin βA (INHβA), are up-regulated in late secretory endometrium (6). To determine whether TGFβ mRNA is regulated in the endometrium during the menstrual cycle, TGFβ1, -2, and -3 gene expression was examined in the same tissues as MMP (Fig. 2). All three TGFβ family members were highly expressed in human endometrial tissue (cycle threshold values of 22–26 using 25 ng RNA for amplification). Whereas TGFβ3 was not regulated in endometrium, TGFβ1 and TGFβ2 were increased significantly in the premenstrual phase (Fig. 2).
Fig. 2.
Regulation of TGF family members in human endometrium. Levels of TGFβ1 (A), TGFβ2 (B), and TGFβ3 (C) mRNA were quantified using quantitative PCR and expressed relative to that of GAPDH as a function of menstrual cycle day. Error bars indicate three to four tissues per day. Data in D–F represent mean ± sem of tissues obtained in proliferative (Prol) (n = 6), early secretory (sec) (n = 5), late secretory (n = 5), and premenstrual (n = 4) phase. *, P < 0.05 compared with proliferative or early secretory, ANOVA.
Endometrial tissue is comprised predominantly of glandular epithelial cells underlying stromal cells. Glandular epithelial cells were separated from stroma and cultured for short times (<7 d) without media supplements. To ascertain the cell type that expressed both MMP and TGFβ, gene expression levels were compared in cultured stromal and epithelial cells (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). All TGFβ family members and MMP2, -9, and -11 were enriched in stromal cells with little or no expression in endometrial epithelial cells. These data indicate that TGFβ and MMP are regulated similarly during the menstrual cycle and that stromal cells are the predominant source. To determine the relationship between TGFβ and these proteases, additional studies were conducted with the prototype TGF family member, TGFβ1, in ESC.
Effect of TGFβ1 on MMP gene expression
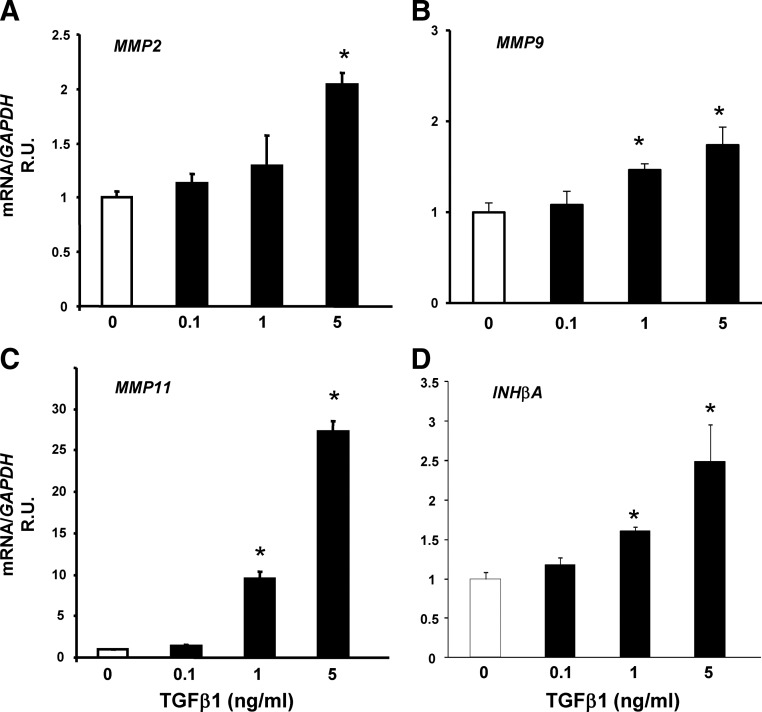
To determine whether TGFβ1 regulates MMP2, MMP9, MMP11, or INHβA in ESC, cells were treated with vehicle or TGFβ1 (0.1–5 ng/ml) for 48 h, and mRNA levels were quantified using quantitative PCR (Fig. 3). TGFβ1 resulted in modest, but significant, increases in MMP2, MMP9, and INHβA mRNA (2- to 2.5-fold). In contrast, TGFβ1 resulted in dramatic up-regulation of MMP11 gene expression (28-fold, Fig. 3C). Together, these data indicate that TGFβ regulates expression of certain MMP and INHβA.
Fig. 3.
Effect of TGFβ1 on MMP2 (A), MMP9 (B), MMP11 (C), and INHβA (D) mRNA in HESC. HESC were treated with TGFβ1 for 48 h (maximal effect). Data represent mean ± sem of four samples in each group. Cells were obtained from menstrual d 25. *, P < 0.05 compared with vehicle. R.U., Relative units.
Effect of progesterone on TGFβ responses
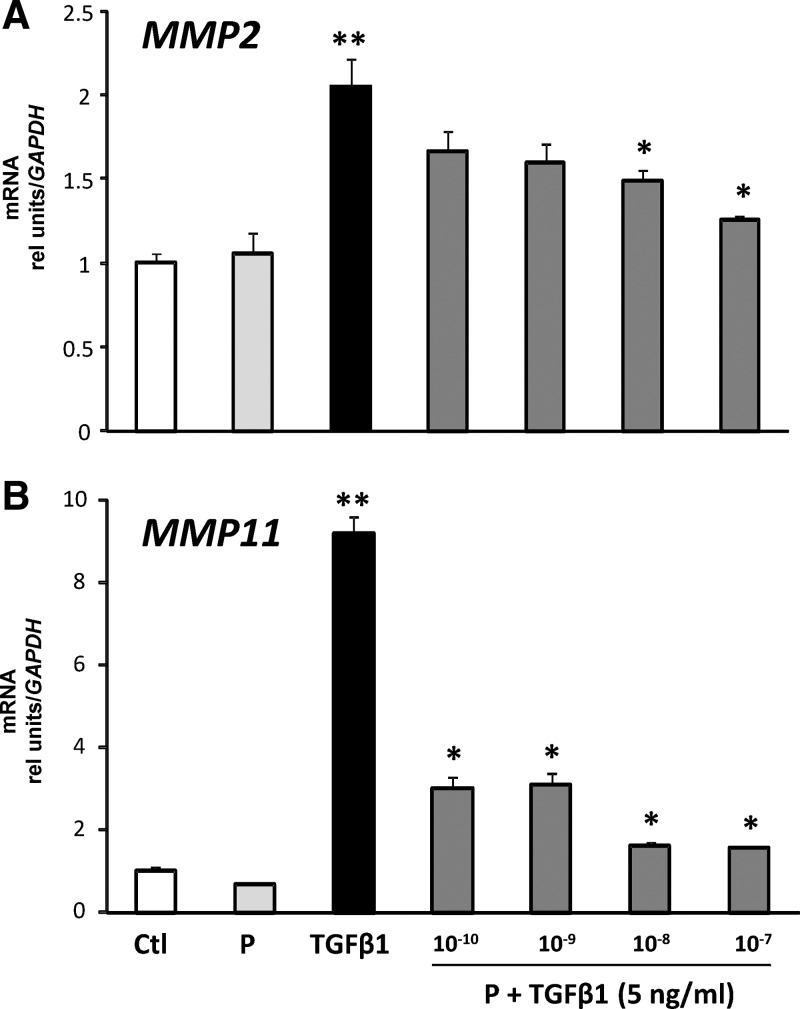
Endometrial changes during the late secretory phase occur in response to progesterone withdrawal. To determine whether progesterone regulates TGFβ responses, ESC were treated with vehicle, progesterone (10−7 m), TGFβ1 (5 ng/ml), or progesterone (10−10 to 10−7 m) plus TGFβ1 for 48 h. Progesterone treatment opposed TGFβ1-mediated increases in MMP2 and MMP11 mRNA in a dose-dependent manner (Fig. 4). The sensitivity of each MMP to progesterone inhibition was not equivalent. MMP11 was exquisitely sensitive to progesterone, whereas inhibition of TGFβ1-induced up-regulation of MMP2 required higher concentrations.
Fig. 4.
Effect of progesterone on TGFβ-induced increases in MMP2 (A) and MMP11 (B) mRNA in HESC. Cells were treated with vehicle [control (Ctl)], progesterone (P, 10−9 m), TGFβ1 (5 ng/ml), or progesterone (10−10 to 10−7 m) plus TGFβ1 for 48 h. Thereafter, mRNA levels were quantified. Data for Ctl, P, TGFβ1, and P (10−9) plus TGFβ1 represent mean ± sem of results from nine experiments conducted with nine cell preps. The inhibitory dose-response curve with P (10−10 to 10−7 m) represent mean ± sem of four samples in each group. **, P < 0.01 compared with control; *, P < 0.01 compared with TGFβ1 alone, ANOVA.
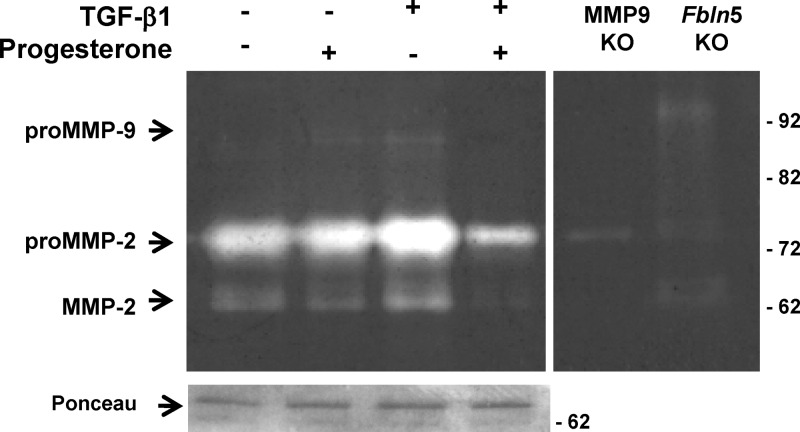
Progesterone also opposed TGFβ1-mediated increases in MMP2 activity as determined by quantitative gelatin zymography (Fig. 5). Interestingly, MMP9 was not increased in TGFβ-treated ESC isolated from tissues in early to midsecretory phase, and in fact, in some cases, TGFβ1 inhibited MMP9 gene expression. Cells isolated from the late secretory and premenstrual phases, however, were responsive to TGFβ1 with significant increases in MMP9 gene expression (Supplemental Fig. 2). These results indicate that ESC in culture (P1) were responsive to progesterone and that although progesterone alone had little effect on baseline MMP2 or MMP11, TGFβ-induced effects were opposed by progesterone treatment.
Fig. 5.
Progesterone and TGFβ1 regulate pro- and active MMP2 activity. Gelatin zymogram of ESC from two preparations (5 μg/lane) treated with vehicle (control), progesterone (10−7 m), TGFβ1 (5 ng/ml), or progesterone plus TGFβ1 for 48 h. Gelatin zymography revealed that progesterone inhibited whereas TGFβ1 increased both pro- and active MMP2. Vaginal tissues from Mmp9 and Fbln5 knockout (KO) mice were used as negative and positive controls.
Functional PR are required to inhibit TGFβ responses
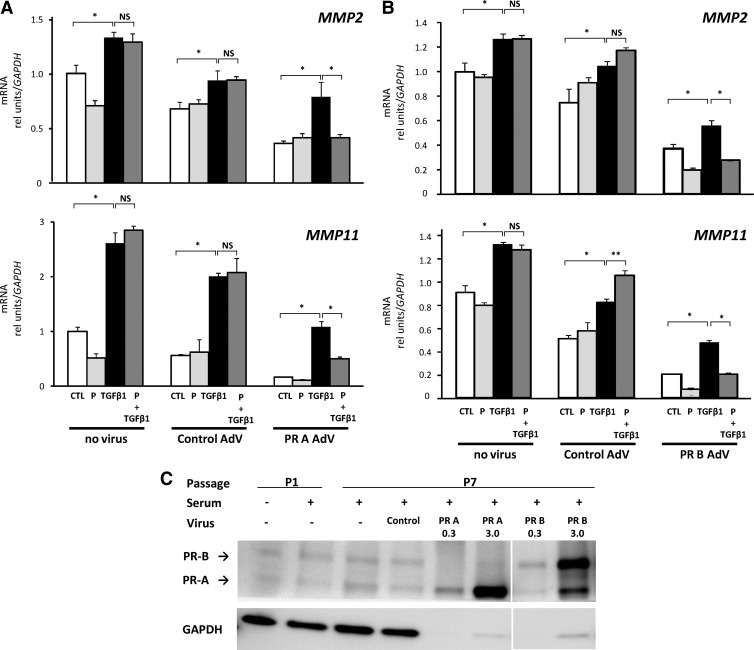
ESC in advanced passage (P7) were used to determine whether nuclear PR are essential in mediating inhibition of TGFβ1 responses. These cells are well known to lose progesterone responsiveness as a function of time in culture and number of passages. Ectopic overexpression of PR-A or PR-B was accomplished with adenoviral constructs. Cells treated with control adenovirus or noninfected cells were used as controls (Fig. 6). In control advanced-passage cells, TGFβ1 induced increased gene expression of MMP2, MMP11, and TGFβ1. Unlike cells in early passage (P1), however, progesterone failed to inhibit these responses. Ectopic overexpression of PR-A or PR-B (Fig. 6) restored progesterone-induced inhibition of TGFβ1 responses. Furthermore, overexpression of either PR, even without ligand, resulted in decreased baseline expression of MMP2, MMP11, and TGFβ1. Overexpression of PR was confirmed by immunoblotting (Fig. 6B). It is interesting to note that although cells in advanced passage fail to respond to progesterone, PR levels seem to be maintained, suggesting that coactivators or repressors are in limited supply in these cells and that overexpression of PR is required to regain progesterone responses. Nonetheless, overall, these results indicate that PR function is required for inhibition of TGFβ1 responses.
Fig. 6.
Ectopic expression of PR isoforms restores progesterone-induced inhibition of TGFβ1 responses in advanced-passage HESC. A and B, Stromal cells in P7 were treated with no virus or adenovirus (AdV) constructs expressing control, PR-A (A), or PR-B (B). After infections, cells were treated with vehicle [control (CTL)], progesterone (P, 10−9 m), TGFβ1 (5 ng/ml) or P plus TGFβ1 for 48 h, and expression of MMP2 and MMP11 were quantified relative to control cells without virus. Data for CTL, P, TGFβ1, and P (10−9 m) plus TGFβ1 represent mean ± sem; n = 4 in each condition. NS, Not significant; *, P < 0.05; **, P < 0.05 compared with TGFβ1 alone. C, Immunoblot analysis of PR in ESC (30 μg/lane) in P1, P7, or P7 treated with AdV for PR-A or PR-B (0.3 or 3.0 μg/lane).
PR gene expression varies across the menstrual cycle
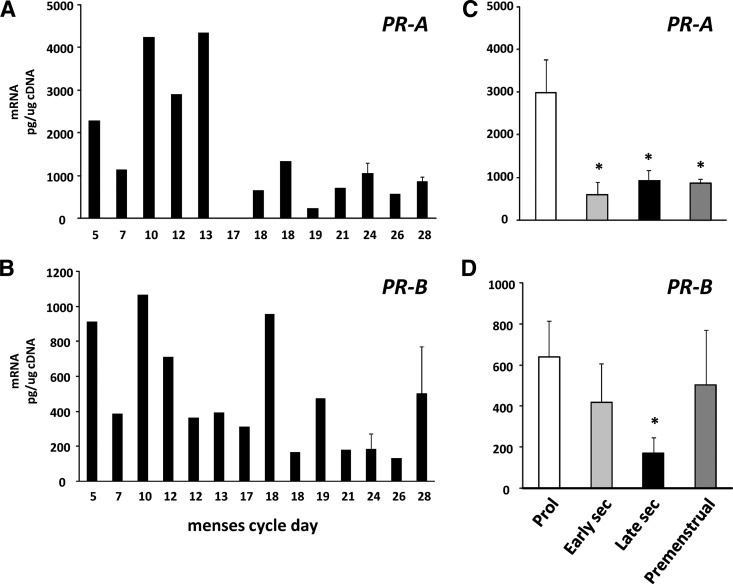
The finding that PR was required for inhibition of TGFβ responses led us to quantify gene expression levels of PR-A and -B in endometrial tissue across the menstrual cycle (Fig. 7). To do so, levels of PR-B were quantified in picograms per microgram of cDNA using standard curves of PR-B. Total PR was quantified (picograms per microgram cDNA) using primers that recognize the C terminus with identical amplification efficiencies as PR-B. PR-B was then subtracted from total PR to estimate PR-A. Both PR-A and PR-B were increased in the proliferative phase relative to the secretory phase. The data indicate that, with some exceptions, both isoforms appear to be regulated synchronously in human endometrium. These results must be interpreted with caution, however, because regulation in stromal and epithelial cellular compartments may be unique relative to PR expression in whole tissues.
Fig. 7.
Regulation of PR isoforms in human endometrial tissues. Levels of PR-A (A) and PR-B (B) were quantified using quantitative PCR and standard curves of PR cDNA. Levels of PR-B were subtracted from total PR to approximate levels of PR-A relative to that of GAPDH as a function of menstrual cycle day. Error bars indicate three to four tissues per day. Data in C and D represent mean ± sem of tissues obtained in proliferative (Prol) (n = 6), early secretory (sec) (n = 5), late secretory (n = 5), and premenstrual (n = 4) phase. *, P < 0.05 compared with proliferative.
Effect of TGFβ1 on PR
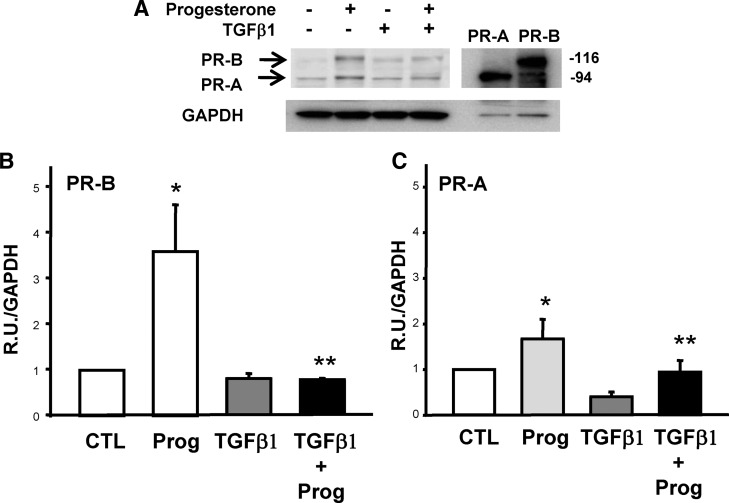
Finally, we determined whether TGFβ1 altered PR expression in ESC (Fig. 8). Interestingly, progesterone treatment resulted in increased expression of both PR-A and -B. Treatment with TGFβ1 alone had little effect on PR expression. TGFβ1, however, opposed progesterone-induced increases in PR expression in HESC. Together, these results suggest that TGFβ1 may serve as an endogenous antiprogestin in ESC.
Fig. 8.
Effect of TGFβ1 on PR expression in HESC. A, Representative immunoblot of PR isoforms in HESC treated with progesterone, TGFβ1, or both for 48 h. Maximal effects were observed with progesterone (10−7 m) and TGFβ1 (5 ng/ml) for 48 h (not shown). GAPDH was used as a loading control. Fibroblasts infected with PR-A or PR-B adenovirus were used as positive controls. B and C, Data represent mean ± sem of relative signal intensities in four immunoblots conducted with samples from four different cell preps. Vehicle-treated control (CTL) cells served as baseline expression of PR-B (B) and PR-A (C). *, P < 0.01 compared with CTL (paired); **, P < 0.05 compared with progesterone (Prog).
Discussion
The ECM is formed from secreted proteins and glycoproteins and forms the ground substance outside cells in all tissues. The ECM appears to play an important role in the cell-cell interactions (22) during menstruation, which is characterized by hemorrhagic shedding of the superficial layer of endometrium as a result of ECM breakdown. This process is associated with expression of MMP (23), a family of more than 20 related enzymes that degrade a number of ECM components in numerous biological processes. The MMP system controls the following aspects of reproductive function: follicular development, ovulation, menstruation, and implantation. In this study, we found that of the two major gelatinases, MMP2 is predominant in endometrium and is increased significantly in the late secretory phase. MMP11 (i.e. stromelysin III) was increased dramatically in the premenstrual phase, falling to baseline levels during the proliferative phase.
Of the numerous MMP described, four (MMP2, MMP9, MMP13, and MMP14) have demonstrable TGFβ-activating capacity (24). TGFβ superfamily members regulate a plethora of diverse biological functions (25–28) and are closely associated with tissue remodeling events and reproductive processes. TGFβ exists as five different isoforms, three of which (TGFβ1, -β2, and -β3) are expressed in humans. Here, we confirmed that, in the endometrium, TGFβ1 is localized to stromal cells (29), and endometrial TGFβ1 mRNA is significantly increased in the midsecretory, late secretory, and menstrual phases compared with proliferative and early secretory phases (30). INHβA is a member of the TGFβ superfamily, is up-regulated 9-fold in late secretory endometrium (6), and binds to and activates TGFβ receptors. MMP2 and MMP9 cleave latency-associated peptide of TGFβ, thereby releasing active TGFβ (31, 32). A detailed study examining global gene expression profiles in endometrial tissues from various stages of the menstrual cycle indicated that expression of several TGFβ-related genes was increased during the late secretory phase (6). Thus, it is possible that protease activation in the late luteal phase may cleave latent TGFβ-binding protein and activate TGFβ signaling in endometrial cells. In agreement with this notion is that a number of studies have demonstrated that progesterone restrains MMP secretion in several tissues (33–35). Thus, a decline in progesterone levels after the midluteal phase may cause up-regulation of proteases that release active TGFβ. In this study, however, progesterone did not have profound effects on baseline levels of MMP2 or MMP11 in ESC. Rather, progesterone mitigated TGFβ-induced increases in both MMP2 and MMP11. Furthermore, and consistent with the findings of Kane et al. (5), TGFβ1 led to decreased PR expression in stromal cells. These findings suggest that declining progesterone levels in the luteal phase may result in loss of the inhibitory influence of progesterone on TGFβ-mediated up-regulation of MMP2 and MMP11. Because MMP2 was less sensitive to progesterone inhibition, decreases in progesterone levels after the midluteal peak may augment MMP2. MMP2, then, may augment TGFβ activity through cleavage of its latent binding protein. Consequently, TGFβ reduces PR expression, thereby leading to an overall deteriorating progestational environment, MMP9 and MMP11 protease activation, and destabilization of endometrial integrity.
The PR is a member of the superfamily of ligand-activated transcription factors that bind to sequence-specific sites in the promoters of target genes. PR exists as two major isoforms, PR-A (94 kDa) and PR-B (114 kDa) (36, 37), both of which are expressed in human endometrium (38). In the human, expression of PR varies temporally and spatially, in both functional and basal regions and within the epithelial and stromal compartments, across the normal menstrual cycle (39–41). Using standard curves of PR cDNA, our data estimated that PR-A was the dominant nuclear hormone receptor in endometrial tissue and that both PR-A and -B were down-regulated in the late luteal phase, possibly by TGFβ. The variable expression of PR-B in premenstrual tissues was due to high expression in one of four samples in this group.
The relationship between progesterone and TGFβ, however, is complex. For example, both progesterone and TGFβ have been shown to repress MMP3 and MMP7 (1, 42). Progesterone was reported to inhibit TGFβ1 mRNA and protein expression in endometrial explants (43). Earlier reports, however, showed that progesterone plus estradiol stimulated TGFβ1 (30) and that progesterone increased TGFβ1 but decreased TGFβ3 mRNA levels in stromal cells (14). Different concentrations of hormones may explain these diverse results (44). In this study, we approximated physiological doses of progesterone and found that progesterone, alone, increased expression of its own nuclear hormone receptor and inhibited TGFβ-mediated up-regulation of MMP2 and -11. Smad signaling has been shown to mediate TGFβ1-mediated loss of PR in stromal cells (5). Studies are ongoing to determine the mechanisms by which PR affects SMAD signaling in the endometrium.
MMP11 is an unusual member of the MMP family. Despite intense efforts since its first characterization 15 yr ago, its role and target substrates in different diseases remain largely unknown. Although mice with null mutations in MMP11 display no phenotype, analysis of different tumorigenesis models indicates that MMP11 promotes tumor development. In contrast with other MMP, MMP11 is unable to degrade any major ECM component. Furthermore, most MMP are secreted as inactive propeptides requiring activation in the extracellular space by proteases, such as plasmin or other MMP. MMP11, however, can be processed directly to its enzymatic active form by an obligate intracellular proteolytic event involving the Golgi-associated proteinase furin. MMP11 may thus play a unique role in tissue remodeling processes. Here, we found, for the first time, that TGFβ1 increases MMP11 gene expression dramatically and that progesterone is a potent inhibitor of TGFβ1-induced increases in MMP11. The extreme sensitivity of MMP11 to progesterone suppression may lead to its up-regulation only during the premenstrual phase, declining to baseline during the early follicular phase, whereas MMP2 is increased during progesterone withdrawal. Other paracrine and matrix components may regulate these genes to orchestrate the complex phenomenon of menstruation and endometrial repair. These possibilities are currently under investigation. Nonetheless, results from this investigation suggest that the opposing effects of progesterone and TGFβ1 are important in regulating the cyclic changes in endometrial ECM remodeling during the menstrual cycle.
Supplementary Material
Acknowledgments
We thank Ms. Valencia Hoffman and the Human Tissue and Biological Core Laboratory supported by NIH HD11149 for tissue acquisition. We also express our gratitude to the Gynecology Core physicians for assistance in collection of tissue and to Mr. Jesus Acevedo and Mr. Patrick Keller for technical advice.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ECM
- Extracellular matrix
- HESC
- human endometrial stromal cell
- INHβA
- inhibin βA
- MMP2
- matrix metalloproteinase 2
- PR
- progesterone receptor
- TBST
- Tris-buffered saline with Tween 20.
References
- 1. Bruner-Tran KL, Eisenberg E, Yeaman GR, Anderson TA, McBean J, Osteen KG. 2002. Steroid and cytokine regulation of matrix metalloproteinase expression in endometriosis and the establishment of experimental endometriosis in nude mice. J Clin Endocrinol Metab 87:4782–4791 [DOI] [PubMed] [Google Scholar]
- 2. Bruner-Tran KL, Rier SE, Eisenberg E, Osteen KG. 1999. The potential role of environmental toxins in the pathophysiology of endometriosis. Gynecol Obstet Invest 48(Suppl 1):45–56 [DOI] [PubMed] [Google Scholar]
- 3. Cornet PB, Picquet C, Lemoine P, Osteen KG, Bruner-Tran KL, Tabibzadeh S, Courtoy PJ, Eeckhout Y, Marbaix E, Henriet P. 2002. Regulation and function of LEFTY-A/EBAF in the human endometrium. mRNA expression during the menstrual cycle, control by progesterone, and effect on matrix metalloprotineases. J Biol Chem 277:42496–42504 [DOI] [PubMed] [Google Scholar]
- 4. Osteen KG, Bruner-Tran KL, Ong D, Eisenberg E. 2002. Paracrine mediators of endometrial matrix metalloproteinase expression: potential targets for progestin-based treatment of endometriosis. Ann NY Acad Sci 955:139–146; discussion 157–158, 396–406 [DOI] [PubMed] [Google Scholar]
- 5. Kane N, Jones M, Brosens JJ, Saunders PT, Kelly RW, Critchley HO. 2008. Transforming growth factor-β1 attenuates expression of both the progesterone receptor and Dickkopf in differentiated human endometrial stromal cells. Mol Endocrinol 22:716–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, Le Shay N, Nezhat CN, Kempson R, Lessey BA, Nayak NR, Giudice LC. 2006. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology 147:1097–1121 [DOI] [PubMed] [Google Scholar]
- 7. Woessner JF., Jr 1991. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J 5:2145–2154 [PubMed] [Google Scholar]
- 8. Emonard H, Grimaud JA. 1990. Matrix metalloproteinases. A review. Cell Mol Biol 36:131–153 [PubMed] [Google Scholar]
- 9. Tabibzadeh S. 1996. The signals and molecular pathways involved in human menstruation, a unique process of tissue destruction and remodelling. Mol Hum Reprod 2:77–92 [DOI] [PubMed] [Google Scholar]
- 10. Salamonsen LA. 1996. Matrix metalloproteinases and their tissue inhibitors in endocrinology. Trends Endocrinol Metab 7:28–34 [DOI] [PubMed] [Google Scholar]
- 11. Davies S, Dai D, Feldman I, Pickett G, Leslie KK. 2004. Identification of a novel mechanism of NF-[κ]B inactivation by progesterone through progesterone receptors in Hec50co poorly differentiated endometrial cancer cells: induction of A20 and ABIN-2. Gynecol Oncol 94:463–470 [DOI] [PubMed] [Google Scholar]
- 12. Allport VC, Pieber D, Slater DM, Newton R, White JO, Bennett PR. 2001. Human labour is associated with nuclear factor-κB activity which mediates cyclo-oxygenase-2 expression and is involved with the ‘functional progesterone withdrawal’. Mol Hum Reprod 7:581–586 [DOI] [PubMed] [Google Scholar]
- 13. Kalkhoven E, Wissink S, van der Saag PT, van der Burg B. 1996. Negative interaction between the RelA(p65) subunit of NF-κB and the progesterone receptor. J Biol Chem 271:6217–6224 [DOI] [PubMed] [Google Scholar]
- 14. Arici A, MacDonald PC, Casey ML. 1996. Modulation of the levels of transforming growth factor β messenger ribonucleic acids in human endometrial stromal cells. Biol Reprod 54:463–469 [DOI] [PubMed] [Google Scholar]
- 15. Casey ML, MacDonald PC. 1996. Transforming growth factor-β inhibits progesterone-induced enkephalinase expression in human endometrial stromal cells. J Clin Endocrinol Metab 81:4022–4027 [DOI] [PubMed] [Google Scholar]
- 16. Casey ML, Mibe M, MacDonald PC. 1993. Regulation of parathyroid hormone-related protein gene expression in human endometrial stromal cells in culture. J Clin Endocrinol Metab 77:188–194 [DOI] [PubMed] [Google Scholar]
- 17. Casey ML, Smith JW, Nagai K, MacDonald PC. 1993. Transforming growth factor-β1 inhibits enkephalinase (EC 3.4.24.11) gene expression in human endometrial stromal cells and sex skin fibroblasts in culture. J Clin Endocrinol Metab 77:144–150 [DOI] [PubMed] [Google Scholar]
- 18. Noyes R, Hertig A, Rock J. 1950. Dating the endometrial biopsy. Fertil Steril 1:3–25 [DOI] [PubMed] [Google Scholar]
- 19. Nasu K, Matsui N, Narahara H, Tanaka Y, Miyakawa I. 1998. Effects of interferon-γ on cytokine production by endometrial stromal cells. Hum Reprod 13:2598–2601 [DOI] [PubMed] [Google Scholar]
- 20. Wieslander CK, Rahn DD, McIntire DD, Acevedo JF, Drewes PG, Yanagisawa H, Word RA. 2009. Quantification of pelvic organ prolapse in mice: vaginal protease activity precedes increased MOPQ scores in fibulin 5 knockout mice. Biol Reprod 80:407–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li XH, Kishore AH, Dao D, Zheng W, Roman CA, Word RA. 2010. A novel isoform of microphthalmia-associated transcription factor inhibits IL-8 gene expression in human cervical stromal cells. Mol Endocrinol 24:1512–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aplin JD, Charlton AK, Ayad S. 1988. An immunohistochemical study of human endometrial extracellular matrix during the menstrual cycle and first trimester of pregnancy. Cell Tissue Res 253:231–240 [DOI] [PubMed] [Google Scholar]
- 23. Kokorine I, Marbaix E, Henriet P, Okada Y, Donnez J, Eeckhout Y, Courtoy PJ. 1996. Focal cellular origin and regulation of interstitial collagenase (matrix metalloproteinase-1) are related to menstrual breakdown in the human endometrium. J Cell Sci 109(Pt 8):2151–2160 [DOI] [PubMed] [Google Scholar]
- 24. Jenkins G. 2008. The role of proteases in transforming growth factor-β activation. Int J Biochem Cell Biol 40:1068–1078 [DOI] [PubMed] [Google Scholar]
- 25. Ignotz RA, Massagué J. 1986. Transforming growth factor-β stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem 261:4337–4345 [PubMed] [Google Scholar]
- 26. Desmoulière A, Geinoz A, Gabbiani F, Gabbiani G. 1993. Transforming growth factor-β1 induces α-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol 122:103–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lewis MP, Lygoe KA, Nystrom ML, Anderson WP, Speight PM, Marshall JF, Thomas GJ. 2004. Tumour-derived TGF-β1 modulates myofibroblast differentiation and promotes HGF/SF-dependent invasion of squamous carcinoma cells. Br J Cancer 90:822–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shephard P, Martin G, Smola-Hess S, Brunner G, Krieg T, Smola H. 2004. Myofibroblast differentiation is induced in keratinocyte-fibroblast co-cultures and is antagonistically regulated by endogenous transforming growth factor-β and interleukin-1. Am J Pathol 164:2055–2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Johnson MC, Torres M, Alves A, Bacallao K, Fuentes A, Vega M, Boric MA. 2005. Augmented cell survival in eutopic endometrium from women with endometriosis: expression of c-myc, TGF-β1 and bax genes. Reprod Biol Endocrinol 3:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Casslén B, Sandberg T, Gustavsson B, Willén R, Nilbert M. 1998. Transforming growth factor β1 in the human endometrium. Cyclic variation, increased expression by estradiol and progesterone, and regulation of plasminogen activators and plasminogen activator inhibitor-1. Biol Reprod 58:1343–1350 [DOI] [PubMed] [Google Scholar]
- 31. Yu Q, Stamenkovic I. 2000. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-β and promotes tumor invasion and angiogenesis. Genes Dev 14:163–176 [PMC free article] [PubMed] [Google Scholar]
- 32. Ge G, Greenspan DS. 2006. BMP1 controls TGFβ1 activation via cleavage of latent TGFβ-binding protein. J Cell Biol 175:111–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang J, Hampton AL, Nie G, Salamonsen LA. 2000. Progesterone inhibits activation of latent matrix metalloproteinase (MMP)-2 by membrane-type 1 MMP: enzymes coordinately expressed in human endometrium. Biol Reprod 62:85–94 [DOI] [PubMed] [Google Scholar]
- 34. Di Nezza LA, Jobling T, Salamonsen LA. 2003. Progestin suppresses matrix metalloproteinase production in endometrial cancer. Gynecol Oncol 89:325–333 [DOI] [PubMed] [Google Scholar]
- 35. Goldman S, Shalev E. 2006. Difference in progesterone-receptor isoforms ratio between early and late first-trimester human trophoblast is associated with differential cell invasion and matrix metalloproteinase 2 expression. Biol Reprod 74:13–22 [DOI] [PubMed] [Google Scholar]
- 36. Horwitz KB, Francis MD, Wei LL. 1985. Hormone-dependent covalent modification and processing of human progesterone receptors in the nucleus. DNA 4:451–460 [DOI] [PubMed] [Google Scholar]
- 37. Wei LL, Gonzalez-Aller C, Wood WM, Miller LA, Horwitz KB. 1990. 5′-Heterogeneity in human progesterone receptor transcripts predicts a new amino-terminal truncated “C”-receptor and unique A-receptor messages. Mol Endocrinol 4:1833–1840 [DOI] [PubMed] [Google Scholar]
- 38. Attia GR, Zeitoun K, Edwards D, Johns A, Carr BR, Bulun SE. 2000. Progesterone receptor isoform A but not B is expressed in endometriosis. J Clin Endocrinol Metab 85:2897–2902 [DOI] [PubMed] [Google Scholar]
- 39. Lessey BA, Killam AP, Metzger DA, Haney AF, Greene GL, McCarty KS., Jr 1988. Immunohistochemical analysis of human uterine estrogen and progesterone receptors throughout the menstrual cycle. J Clin Endocrinol Metab 67:334–340 [DOI] [PubMed] [Google Scholar]
- 40. Garcia E, Bouchard P, De Brux J, Berdah J, Frydman R, Schaison G, Milgrom E, Perrot-Applanat M. 1988. Use of immunocytochemistry of progesterone and estrogen receptors for endometrial dating. J Clin Endocrinol Metab 67:80–87 [DOI] [PubMed] [Google Scholar]
- 41. Snijders M, de Goeij A, Koudstaal J, Bosman F. 1996. Oestrogen and progestogen receptor content in human endometrium. Eur J Obstet Gynecol Reprod Biol 70:9–10 [DOI] [PubMed] [Google Scholar]
- 42. Bruner KL, Rodgers WH, Gold LI, Korc M, Hargrove JT, Matrisian LM, Osteen KG. 1995. Transforming growth factor β mediates the progesterone suppression of an epithelial metalloproteinase by adjacent stroma in the human endometrium. Proc Natl Acad Sci USA 92:7362–7366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gaide Chevronnay HP, Cornet PB, Delvaux D, Lemoine P, Courtoy PJ, Henriet P, Marbaix E. 2008. Opposite regulation of transforming growth factors-β2 and -β3 expression in the human endometrium. Endocrinology 149:1015–1025 [DOI] [PubMed] [Google Scholar]
- 44. Omwandho CO, Konrad L, Halis G, Oehmke F, Tinneberg HR. 2010. Role of TGF-βs in normal human endometrium and endometriosis. Hum Reprod 25:101–109 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.