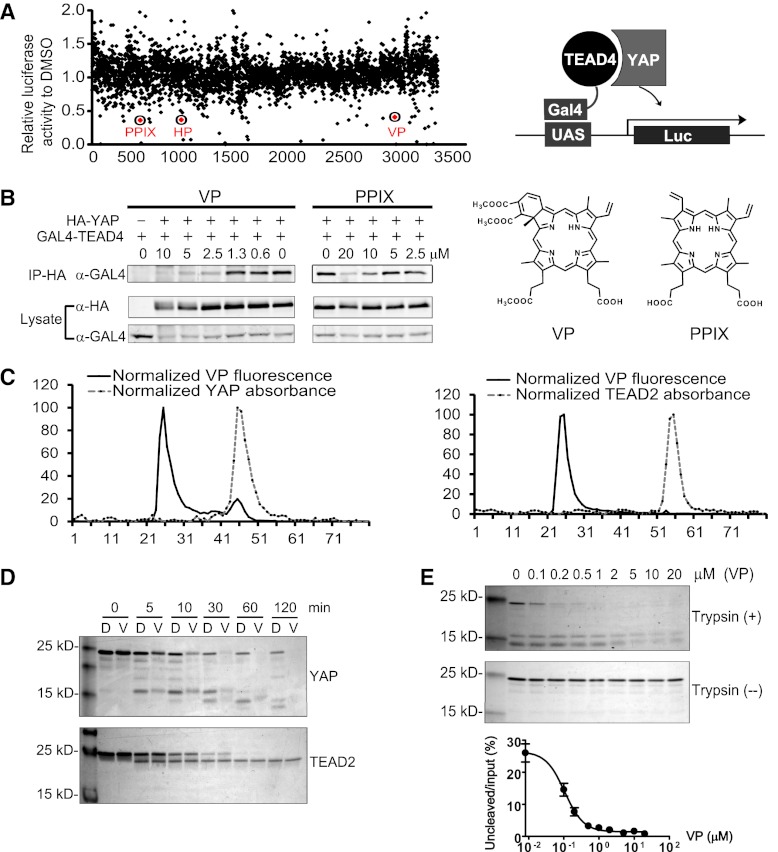
Figure 3.
Identification of VP and related porphyrin compounds as inhibitors of TEAD–YAP interactions. (A) Plot showing distribution of luciferase activity of HEK293 cells expressing Gal4-TEAD4/YAP/UAS-Luc and treated with individual Hopkins Library compounds at 10 μM. All three porphyrin derivatives from the library (PPIX, HP, and VP) were scored as hit compounds as circled. A schematic drawing of the luciferase assay is also shown. (B) Inhibition of TEAD–YAP interaction by VP and PPIX. HEK293 cells expressing Gal4-TEAD4 and HA-YAP were incubated with the indicated concentrations of each chemical, and the presence of Gal4-TEAD in the HA-YAP immunoprecipitates was probed. The chemical structures of VP and PPIX are also shown. (C) VP binds to purified YAP in vitro. Purified YAP (5 μM) (left) or TEAD2 (5 μM) (right) was incubated with VP (15 μM) for 15 min at room temperature. The mixture was then fractioned through a size exclusion column, and the VP fluorescence was measured in each fraction. Note that an appreciable VP fluorescence coeluted with the YAP protein peak (fraction 45, left) but not with the TEAD2 protein peak (fraction 54, right). (D) Effect of VP on trypsin cleavage of YAP or TEAD2. Purified YAP or TEAD2 (5 μM) was preincubated with DMSO (D) or 20 μM VP (V) for 30 min at room temperature prior to trypsin digestion for the indicated time. The cleavage products were analyzed by Coomasie brilliant blue staining after SDS-PAGE. (E) Effect of different concentrations of VP on trypsin cleavage of YAP. YAP (5 μM) was preincubated with various concentrations of VP for 30 min at room temperature, followed by incubation with (+) or without (−) trypsin (0.1 μg/mL) for 1 h on ice. The graph shows quantification of uncleaved versus input YAP at various VP concentrations.