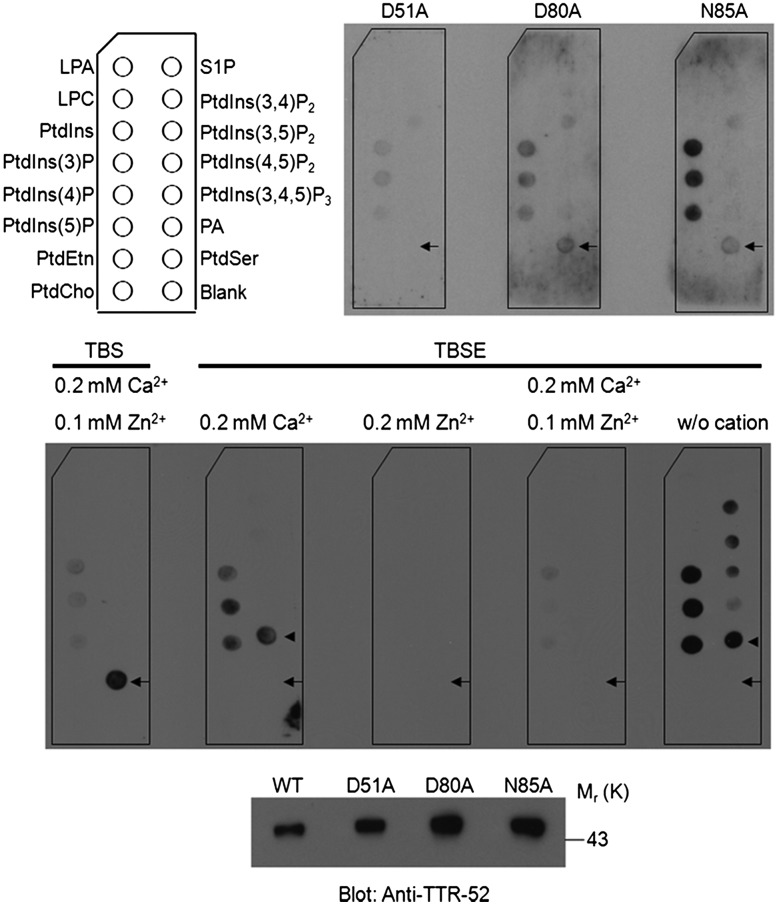
Figure 4.
TTR-52 mutations and cation depletion disrupt PtdSer binding in vitro. (Top panel) Affinity-purified TTR-52-mCherry-Flag binds specifically to PtdSer on a membrane lipid strip, while point mutations D51A, D80A, and N85A greatly decrease the PtdSer-binding ability of TTR-52. (Bottom panel) Amounts of purified TTR-52 proteins used in lipid binding are shown by immunoblotting. (Middle panel) After chelating with 1 mM EDTA, TTR-52 alters the binding affinity of PtdSer to phosphatidic acid, and the ability of TTR-52 to bind to PtdSer was not restored when it was equilibrated in cation solutions. Two independent experiments were performed. (LPA) Lysophosphatidic acid, (LPC) lysophosphatidylcholine, (S1P) sphingosine-1-phosphate, (PA) phosphatidic acid. Arrows indicate PtdSer, and arrowheads indicate PA.