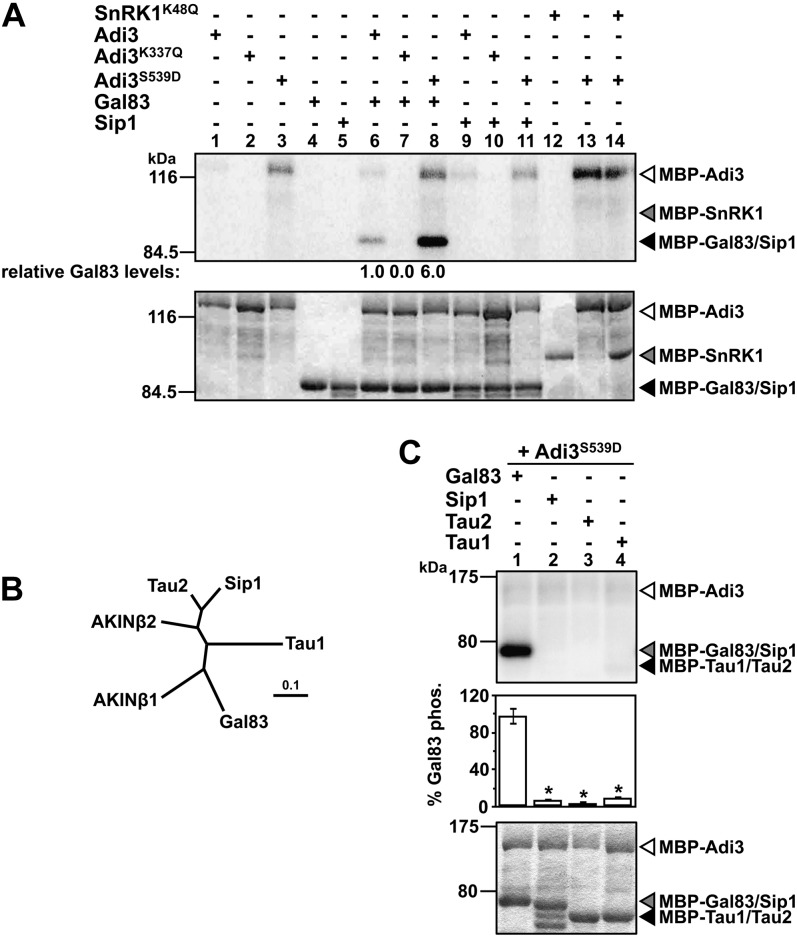
Figure 2.
Adi3 phosphorylates Gal83. In A and C, top panels show phosphor images and bottom panels show Coomassie blue-stained gels. Quantity One software was used to normalize the phosphorylation levels to the protein levels in each assay. A, Analysis of SnRK1 α- and β-subunit phosphorylation by Adi3. Kinase-active and -inactive MBP-Adi3 proteins were tested for phosphorylation of MBP-Gal83, MBP-Sip1, and kinase-inactive MBP-SnRK1K48Q using [γ-32P]ATP in in vitro kinase assays. Gal83 phosphorylation values are reported as a percentage of wild-type Adi3 phosphorylation of Gal83 and are representative of two independent experiments. B, Phylogenetic relationship between tomato and Arabidopsis β-subunits. Proteins were aligned using ClustalW (Larkin et al., 2007), and the tree produced was analyzed using TreeView (Page, 1996). The scale bar indicates the number of amino acid substitutions per site. C, Adi3 only phosphorylates the Gal83 β-subunit. Kinase-active MBP-Adi3S539D was tested for phosphorylation of MBP-Gal83, MBP-Sip1 MBP-Tau1, and MBP-Tau2 as in A. Values are averages of three independent experiments. Error bars represent se. Asterisks indicate significant decreases in β-subunit phosphorylation as compared with Gal83 phosphorylation (Student’s t test, P < 0.01).