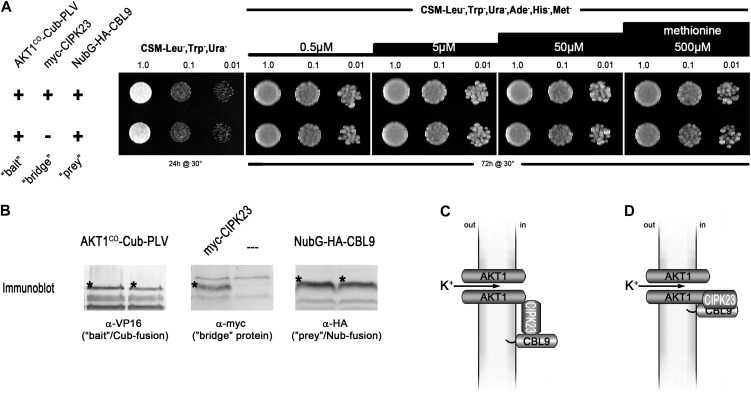
Figure 1.
SUB assay of full-length AKT1 with CIPK23 and CBL9. A, Growth assay of haploid yeast coexpressing the Met-repressible bait construct AKT1co-Cub-PLV (where “co” = codon optimized) and the prey construct NubG-2xHA-CBL9. A construct for a myc-tagged CIPK23 as bridge protein was included in the yeast in the top row but excluded in the yeast of the bottom row. Yeast were dropped at optical density values of 1.0, 0.1, and 0.01 on vector-selective (CSM-Leu-,Trp-,Ura-) and interaction-selective (CSM-Leu-,Trp-,Ura-,Ade-,His-,Met-) media with increasing Met concentrations. Growth was monitored after 24 h for the vector-selective control plates and after 72 h for the actual interaction plates. (Methodical details can be found in Grefen et al. [2009] and Grefen [2012].) B, Western-blot analyses of the two yeast clones, verifying the expression of bait, bridge, and prey fusions. Asterisks mark the bands that correspond to the expected protein sizes of the respective fusion proteins: AKT1co-Cub-PLV = 150.5 kD; myc-CIPK23 = 57.0 kD; NubG-2xHA-CBL9 = 32.1 kD. C, Schematic depiction of the anticipated tripartite interaction of CBL9-CIPK23 and AKT1 (according to Lee et al. [2007]). D, One possible alternative for the interaction that would accord with our observations. PLV, Protein A-LexA-VP16.