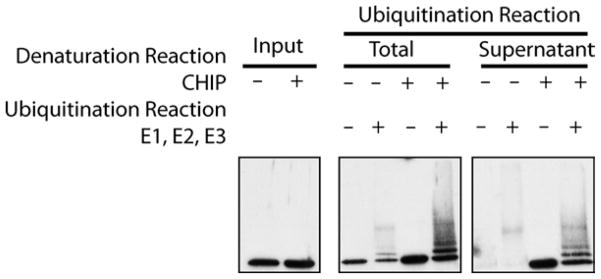
Fig. 2.
Hsp40/Hsp70-independent ubiquitination of denatured luciferase. When CHIP is present during the reaction in which luciferase is heat denaturated by incubation at 42°C for 15 min, the luciferase is maintained in a soluble, ubiquitination-competent state. The total ubiquitination reaction (lanes 3–6) represents the amount of ubiquitination that occurs when CHIP is included or excluded from the denaturation reaction. In lanes 7–10, the denaturation reaction is spun at 20,000 rpm for 10 min at 4°C and the supernatant is removed to a different tube. Ubiquitination enzymes are then added to this supernatant fraction. As apparent from lanes 7 and 8, when luciferase is denatured in the absence of CHIP, the majority aggregates, pellets after centrifugation, and is therefore not detectable in the ubiquitination reactions performed on the supernatant fraction alone. Alternatively, when CHIP is present during the initial denaturation reaction (lanes 9 and 10 ), the CHIP is maintained soluble and efficiently ubiquitinated in the presence of the ubiquitination enzymes.