Summary
We developed a rabbit saccular aneurysm model for coil embolization training. Elastase-induced aneurysms were created successfully in about 80% of the rabbits.
The aneurysms were usually broad in the neck and lengthy. At the 28th postoperative day, the aneurysms were about 1.5 times larger in both width and height than they had been at the 14th day. All aneurysms were successfully embolized with 18-sized electrically detachable (ED) platinum coils. After embolization, almost all aneurysms had a neck remnant. In conclusion, this model is useful not only for learning the technique of coil embolization but also for testing new embolic materials. The rabbit aneurysm model proved to be an efficacious training modality for endovascular coil embolization.
Key words: endovascular training, rabbit, saccular aneurysm
Introduction
The endovascular treatment of intracranial aneurysms with Guglielmi detachable coils (GDC; Boston Scientific, Fremont, CA) has become widely accepted as a safe and less invasive alternative to standard surgical clipping. To become proficient in this technique, the surgeon needs to be knowledgeable of the development of embolic materials and devices, and must receive specialized training. To facilitate this process, we developed a coil embolization training system using the rabbit saccular aneurysmal model 1-3.
Methods
Creation of Aneurysm Model
New Zealand White rabbits were used in this study. After induction of anesthesia with intravenous pentobarbital, the right common carotid artery (CCA) was surgically exposed, and ligated distally with a 3-0 silk suture.
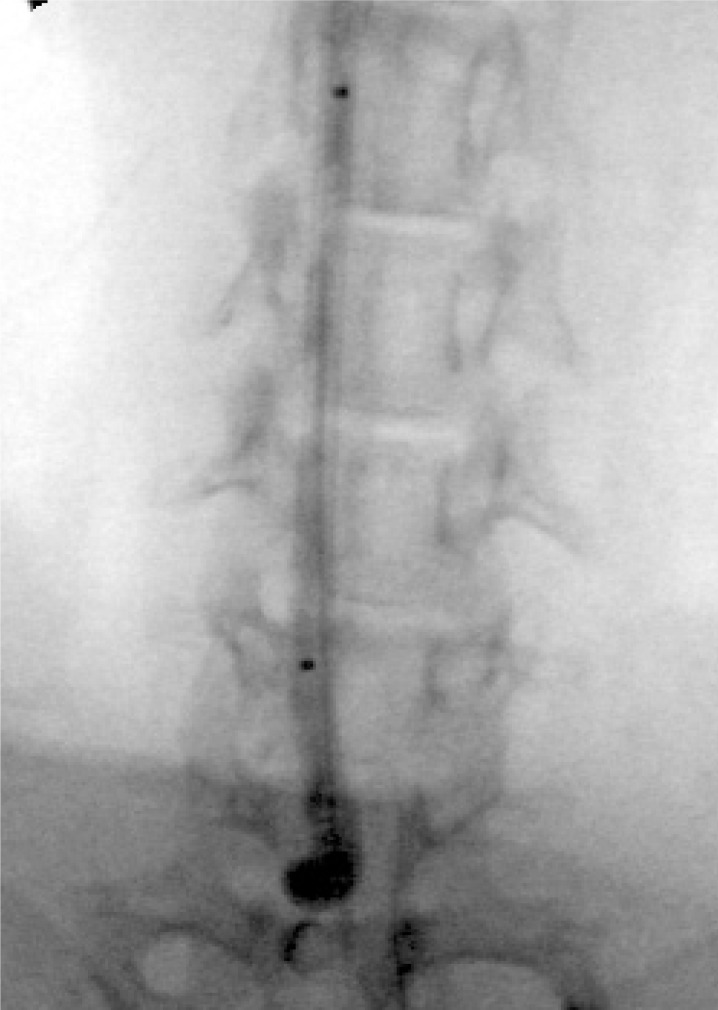
After proximal arterial flow was controlled with a 3-0 silk suture, a small arteriotomy was made and a 4 Fr sheath was introduced retrograde to the CCA. Then, a Pit on Tri-adaptor (Medtronic AVE, Minneapolis, MN) was attached to the sheath. Under fluoroscopic guidance, a Machshield balloon catheter (Kaneka Medix, Osaka, Japan) was advanced into the orifice of the right CCA. Just at the orifice, a balloon was inflated until it became heart-shaped (figure 1).
Figure 1.
Right neck radiograph. At the orifice of the right CCA, a balloon is inflated until it becomes heart-shaped.
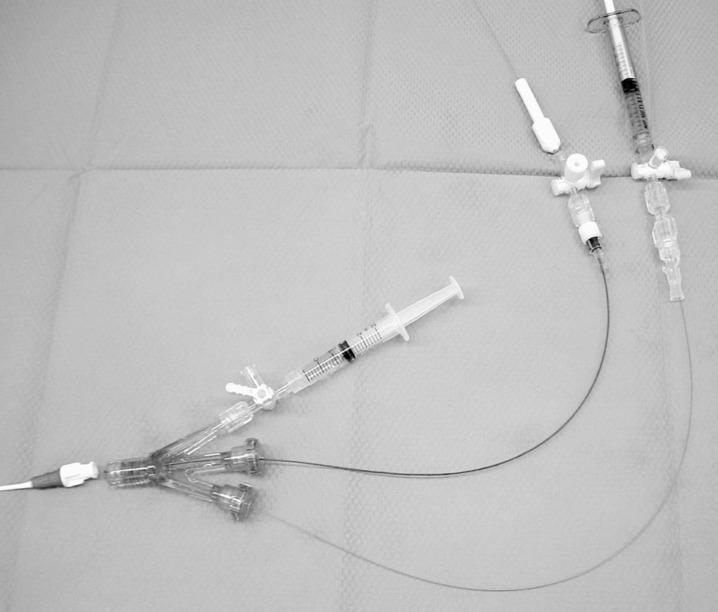
Subsequently, we were able to completely close the space between the balloon and the sheath in the right CCA. A microcatheter was navigated just proximal to the balloon via a Tri-adaptor (figure 2). Then, porcine elastase (Sigma, Saint Louis, MO) was mixed with a non-ionic contrast medium. A total of 1 ml of elas-tase solution was injected from a microcatheter under fluoroscopic guidance. The microcatheter and balloon were removed 15 minutes after incubation of the elastase in the CCA. The sheath was removed and the vessel was ligated proximally. After the procedure, the rabbits were maintained on a normal diet for four weeks.
Figure 2.
Photograph of devices. A Machshield balloon catheter and microcatheter is navigated via a Tri-adaptor.
Follow-up Angiography
We performed follow-up angiograpy 14 and 28 days after surgery. A 24 gauge needle was placed in a right ear vein, 5 ml of contrast medium was injected, and digital subtraction angiography was performed at a rate of four frames per second. Filming was performed at the arterial phase.
Coil Embolization Training
A 4 Fr BHW-shaped catheter with a 0.038 inch inner lumen was used as a guiding catheter. Using the aforementioned technique, a 4 Fr sheath was inserted into the right femoral artery.
After measurement of the aneurysm, a 4 Fr guiding catheter was placed in the right brachiocepharic artery. Then, an 18-sized microcatheter was navigated into the aneurysm; 18-sized electrically detachable (ED) coils (Kaneka Medix, Osaka, Japan) were used in this series.
Results
Development of Aneurysms
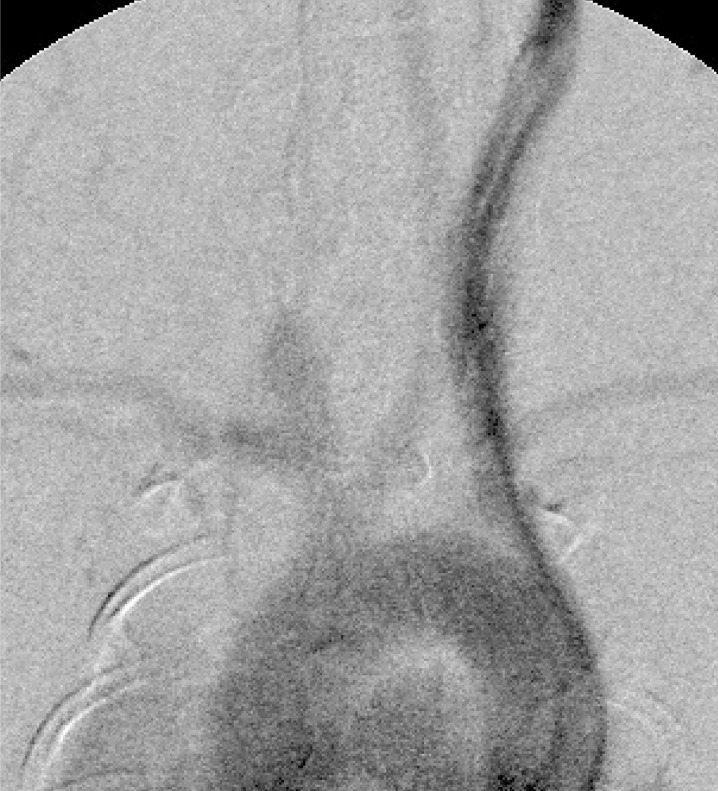
In our series, aneurysms were successfully created in about 80% of the rabbits, and almost all aneurysms were broad in the neck and lengthy. On the 28th postoperative day, the aneurysms were about 1.5 times larger in both width and height than they had been on the 14th day (figure 3).
Figure 3.
Intravenous aortogram 28 days postoperatively. Almost all aneurysms have a broad neck and are lengthy.
Coil Embolization Training
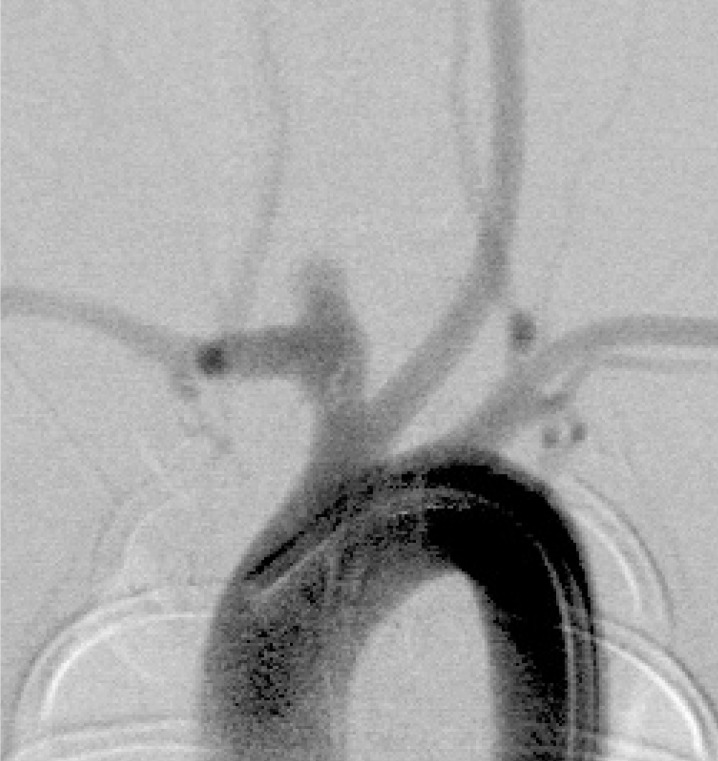
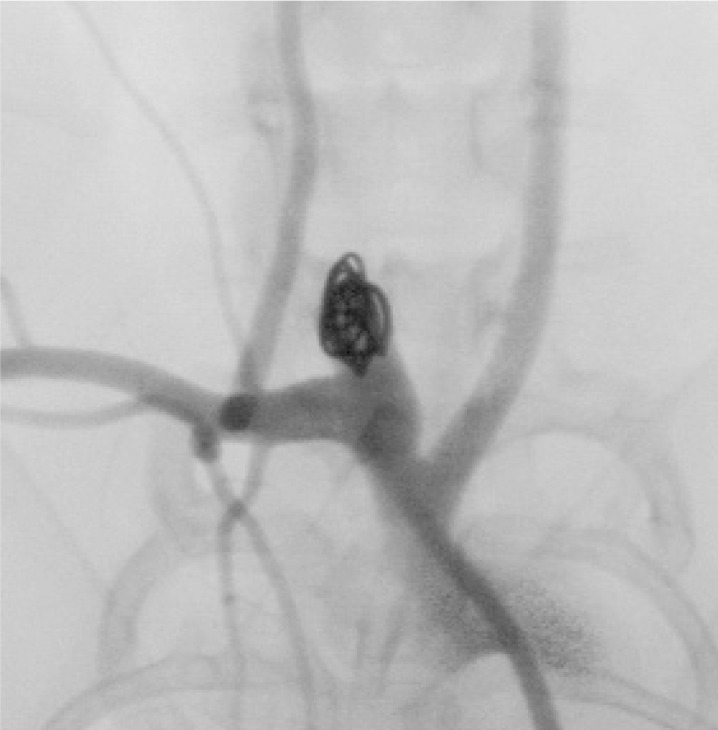
In this series, we successfully embolized all aneurysms with 18-sized ED coils. The coil materials and the detachment system closely approximated the clinical situation. After embolization, almost all aneurysms had a remnant area in the neck; this was due to the shape of the aneurysms together with a limitation of coil variation (figures 4,5).
Figure 4.
Aortogram just before embolization. A broadbased aneurysm is produced at the orifice of the right common carotid artery.
Figure 5.
Angiogram of the right brachicepharic artery. An aneurysm after embolization shows neck remnant.
Discussion
Configuration and Neck Morphology of Aneurysms
At surgery, a balloon was inflated in the shape of a heart to occlude the common carotid artery. After 28 days, angiography revealed that almost all the aneurysms were broad in the neck and lengthy; thus, a neck remnant was present in most cases. For complete embolization, the aneurysm should be round with a narrow neck. Thiex et Al. reported that balloon oc-clusion in the brachiocepharic trunk resulted in broad-based aneurysms, whereas balloon occlusion in the CCA gave rise to a circumscribed aneurysm 4.
Advantages of Coil Embolization Training Using Saccular Aneurysms in Rabbits
In this series, rabbits were chosen for embolization because they are easy to rear and have coagulation system similar to humans. In our training system, the handling of coils and microcatheters was similar to the clinical situation. Although several silicone models have been introduced, the characteristics of both the aneurysmal and vessel wall are different from those of living tissue 5.
Moreover, radiological and histological evaluation was possible after embolization in this series. This model should prove efficacious for training after the introduction of new embolic materials.
Conclusions
The rabbit aneurysm model is useful for coil embolization training; moreover, it is useful for testing new embolization devices. The technique of endovascular coil embolization can be learned using this model.
References
- 1.Fujiwara NH, Cloft HJ, et al. Serial angiography in an elastase-induced aneurysm model in rabbits: evidence for progressive aneurysm enlargement after creation. Am J Neuroradiol. 2001;22:698–703. [PMC free article] [PubMed] [Google Scholar]
- 2.Kallmes DF, et al. New expandable hydrogel-platinum coil hybrid device for aneurysm embolization. Am J neuroradiol. 2002;23:1580–1588. [PMC free article] [PubMed] [Google Scholar]
- 3.Krings T, Moller-Hartmann W, et al. A refined method for creating saccular aneurysms in the rabbit. Neuroradiology. 2003;45:423–429. doi: 10.1007/s00234-003-0976-2. [DOI] [PubMed] [Google Scholar]
- 4.Thiex R, Moller-Hartmenn W, et al. Are the configuration and neck morphology of experimental aneurysms predictable? A technical approach. Neuroradiology. 2004;46:571–576. doi: 10.1007/s00234-004-1218-y. [DOI] [PubMed] [Google Scholar]
- 5.Sugiu K, Martin JB, et al. Artificial cerebral aneurysm model for medical testing, training, and reseach. Neurol Med Chir (Tokyo) 2003;43:69–73. doi: 10.2176/nmc.43.69. [DOI] [PubMed] [Google Scholar]