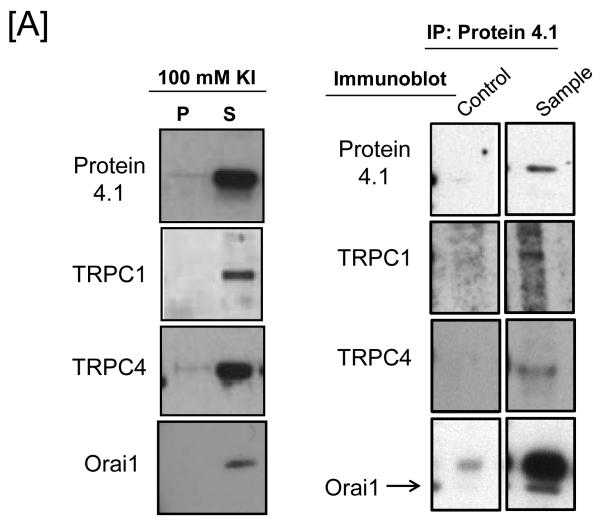
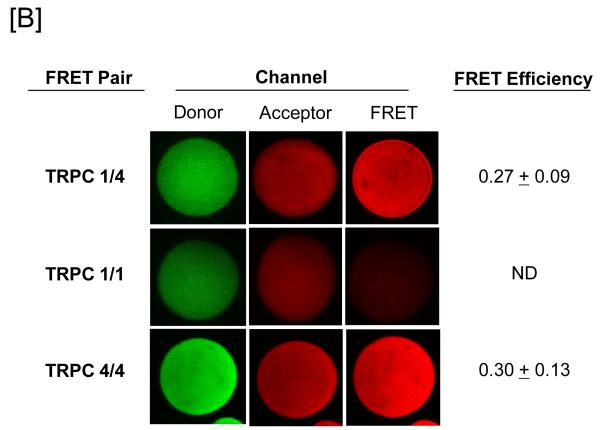
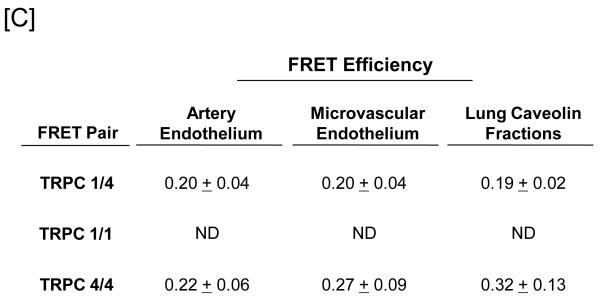
Figure 1. Protein 4.1 interacts with TRPC1 and TRPC4 in vitro and in vivo.
[A] Pulmonary artery endothelial cell membrane/cytoskeleton fractions were detergent extracted in the presence of 100 mM potassium iodide, KI. Pellet and supernatant fractions were separated on SDS-PAGE and immunoblot analysis performed. Protein 4.1, TRPC4, TRPC1, and Orai1 were resolved in the salt-dissociated supernatant (left panel). Protein 4.1 was immunoprecipitated and co-immunoprecipitation of TRPC4, TRPC1, and Orai1 was probed via immunoblot. TRPC4, TRPC1, and Orai1 co-immunoprecipitated with protein 4.1. [B] The protein 4.1-bound TRPC channel possesses TRPC1 and TRPC4 subunits. Protein 4.1-bound immunocomplexes containing the TRPC channel were isolated on agarose beads and treated with cy3-(donor channel) and cy5-(acceptor channel) labeled antibodies at their EC50 concentrations; a single bead is shown. FRET analyses were performed using the sensitized emission approach (FRET channel). FRET pairs refer to the cy3- and cy5-labeled proteins, respectively. FRET efficiency is derived from the summary of a minimum of 9 separate measurements. ND refers to not detected. [C] The TRPC channel FRET signal is identical in vitro and in vivo. The protein 4.1-bound immunocomplex was isolated from pulmonary artery endothelial cells, pulmonary microvascular endothelial cells and lung caveolin-enriched fractions purified from the intact pulmonary circulation. FRET efficiency is derived from the summary of a minimum of 15 separate measurements.