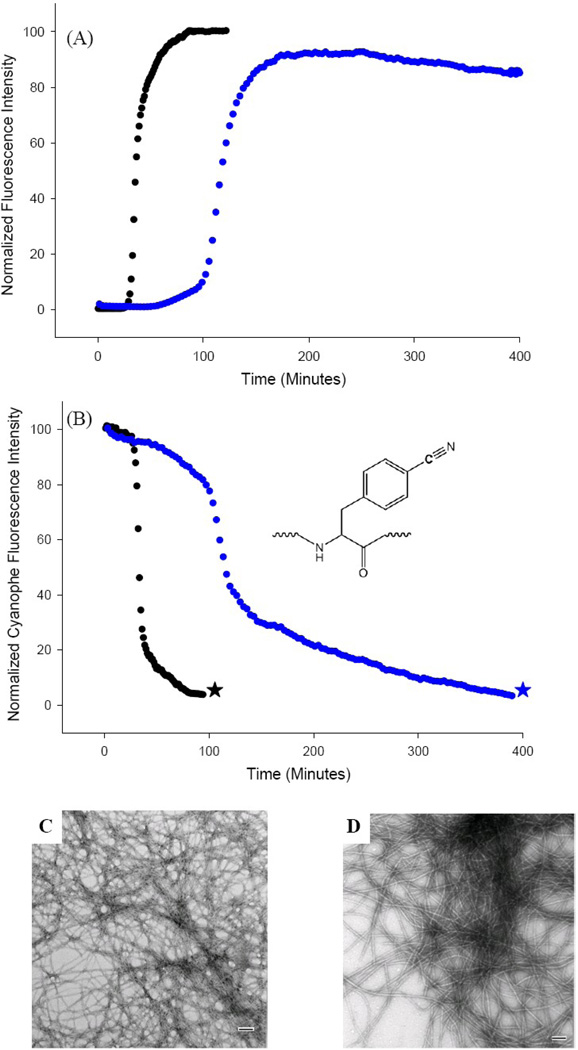
Figure 5.
The S20K mutant of IAPP inhibits amyloid formation by wild type IAPP. (A) Thioflavin-T monitored kinetic experiments. Black: wild type IAPP; Blue: 1:1 mixture of wild type IAPP and S20K-IAPP. (B) p-Cyanophenylalanine fluorescence studies. The black curve is the kinetic trace for F15 p-cyanoPhe IAPP in the absence of inhibitor. The blue curve is the kinetic trace in the presence of a 1:1 ratio of S20K-IAPP. (C) TEM image of amyloid fibers formed by F15 p-cyanoPhe IAPP in the absence of S20K-IAPP (black star). (D) TEM image of amyloid fibers formed by the 1:1 mixture of F15 p-cyanoPhe IAPP and S20K-IAPP (blue star). Experiments were conducted at 25°C, 20 mM Tris-HCl, pH 7.4, 2% HFIP (v/v) with constant stirring. IAPP was at 16 µM, S20K-IAPP when present was also at 16 µM. Scale bars in the TEM images represent 100 nm. F15 p-cyanoPhe IAPP was used as the wild type for both kinetic experiments to allow direct comparison of the thioflavin-T and p-cyanoPhe fluorescence data from the sample. The p-cyanoPhe substitution has been shown not to perturb amyloid formation.