Figure 1.
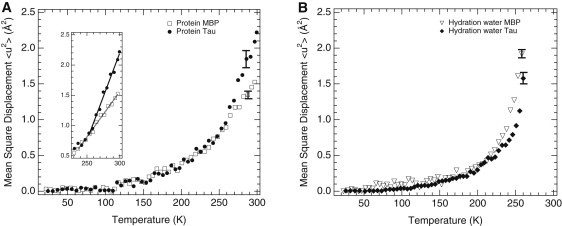
(A) MSDs of side-chain motions as a function of temperature for the IDP tau (solid circles, H-tau-D2O) and the folded MBP (open squares, H-MBP-D2O) as determined by neutron spectroscopy on hydrogenated protein powders hydrated in D2O to ∼0.4 g water/g protein. Effective force constants were 0.185 Nm−1 (MBP) and 0.096 Nm−1 (tau) as extracted from the slopes above 250 K (inset). (B) MSDs of hydration-water motions on the surfaces of tau (solid diamonds; D-tau-H2O) and MBP (open triangles; D-MBP-H2O) as determined on deuterated protein powders hydrated in H2O at ∼0.4 g water/g protein. The MBP data (7) were reexamined for MSD extraction in the same Q2-ranges as for tau. Errors are shown for selected high-temperature data points and are smaller at lower temperatures (not shown). Data were measured on the IN16 spectrometer at the ILL, Grenoble, France.
