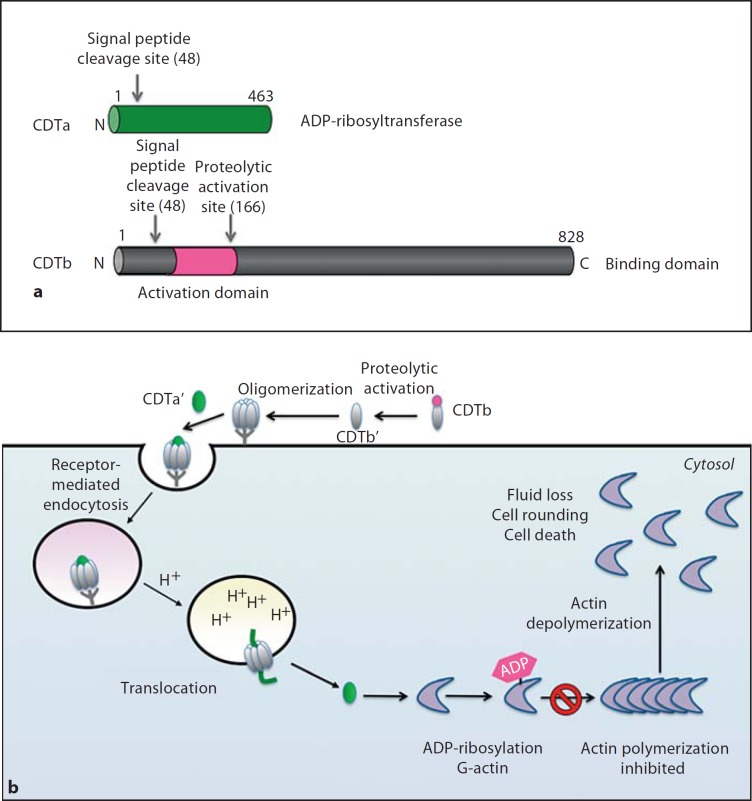
Fig. 3.
aCDT is comprised of 2 polypeptides, CDTa (ADP-ribosyltransferase, green) and CDTb (receptor binding domain, grey). Signal peptide sequences of both polypeptides are shown. CDTb contains a propeptide whose proteolytic removal allows the activated binding domain (CDTb’ in b) to interact with as yet unidentified receptors on target cells. b Intoxication of target cells by CDT. Following proteolytic activation on the target cell surface, the binding domain oligomerizes and binds to cell receptors. Binding results in receptor-mediated endocytosis. During acidification of the endosome, the binding domain undergoes conformational rearrangements that result in pore formation and translocation of the ADP-ribosyltransferase domain into the target cell cytosol in an Hsp90-dependent manner. The ADP-ribosyltransferase domain ADP-ribosylates monomeric G-actin on Arg177, which blocks actin polymerization and ultimately leads to the dissolution of the actin cytoskeleton. CDTa also induces formation of microtubule projections, which may enhance the adherence of C. difficile to the intestinal epithelium.