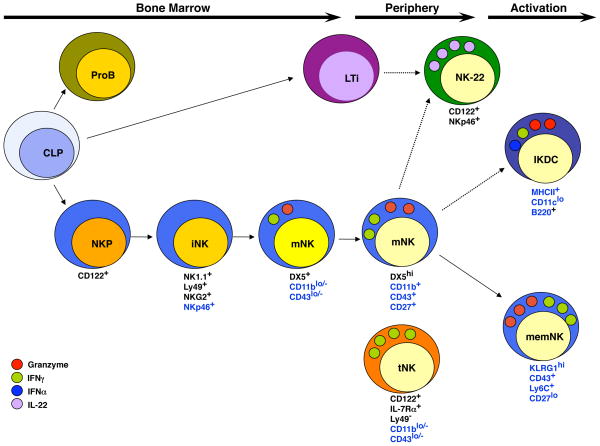
Figure 1.
A Schematic Representation of Natural Killer Cell Development. Conventional NK cell development in the mouse occurs in the bone marrow where common lymphoid progenitors (CLPs) undergo NK cell lineage specification and commitment resulting in CD122+ NK cell progenitors (NKP). Acquisition of activating and inhibitory receptors such as NK1.1, Ly49, NKG2 and NKp46 occurs at the iNK cells stage. Subsequent acquisition of DX5 and CD27 occurs at the transition to the mNK cell stage as the cells undergo IL15-driven expansion. In the periphery mNK cells undergo further maturation as detected by increased expression of CD11b and CD43 and transient up-regulation of CD27 and become efficient at target directed cytolysis and cytokine production. Additional subsets of NK cells can be identified based on differential surface receptor expression including NK22 cells, which express CD122 and NKp46 but, for the most part, not NK1.1 and produce IL22. IKDCs express B220 and low levels of CD11c and MHC Class II but may represent an activation state rather than distinct NK cell fate. Memory NK cells are similar in phenotype to peripheral mNK cells except that they are KLRG1+ and have a heightened capacity for cytolysis and cytokine production. A subset of mNK cells (tNK) that lacks Ly49 receptors, CD11b and CD43 but expresses IL7Rα develops in the thymus and is found primarily in lymph nodes. These cells are inefficient killer cells but produce large quantities of cytokines. Dashed arrows represent lineage relationships that have not yet been fully characterized. Markers noted in black continue to be expressed on the progeny of this cell. Markers noted in blue are differentially expressed.