Abstract
To explore stem cell-mediated neuronal protection through extracellular signaling pathways by transplanted stem cells, we sought to identify potential candidate molecules responsible for neuronal protection using an in vitro co-culture system. Primary fetal rat hippocampal neurons underwent hypoxia (≤1% oxygen) for 96 hours, then were returned to a normoxic condition. The study group then received rat umbilical cord matrix derived stem cells, while the control group received fresh media only. The experimental group showed decreased neuronal apoptosis compared to the control group [44.5 ± 1.6 % vs. 71.0 ± 4.2 % (mean ± SD, p = 0.0005) on Day 5] and higher neuronal survival (4.9 ± 1.2 cells/100x field vs. 2.2 ± 0.3, p = 0.02 on Day 5). Among 90 proteins evaluated using a protein array, stem cell co-culture media showed increased protein secretion of TIMP-1 (5.61 fold), TIMP-2 (4.88), CNTF-Rα (3.42), Activin A (2.20), Fractalkine (2.04), CCR4 (2.02), and decreased secretion in MIP-2 (0.30 fold), AMPK α1 (0.43), TROY (0.48), and TIMP-3 (0.50). This study demonstrated that co-culturing stem cells with primary neurons in vitro decreased neuronal cell death after hypoxia with significantly altered protein secretion. The results suggest that stem cells may offer neuronal protection through extracellular signaling.
Keywords: stem cell, neuronal protection, rat, protein array, hypoxia
INTRODUCTION
Neuronal protection and repair are of paramount importance for patients suffering from ischemic brain injury. Despite extensive research for therapeutic modalities, very few treatments are available today to effectively restore lost neuronal cells and their function after stroke.
Stem cell therapy in an injured brain or a brain at risk of ischemic injury has emerged as a new therapeutic option (5,16). Stem cell transplantation has been demonstrated to provide neuroprotection in rodent models (6,9,21,37). Integration of the transplanted stem cells into the host brain to restore neuronal circuitry has been the major theoretical premise of cell transplantation therapy and the primary reason for the use of neuronal progenitor cells. Recently, however, several studies support the notion of a “bystander” role played by stem cells, demonstrating neurological functional recovery in rodent stroke models without the evidence of integration of transplanted stem cells in the brain at risk (6,19,20,37). This novel mechanism of stem cell repair could be expressed via elicitation of synergistic extracellular signaling pathway(s), which may be responsible for functional and histological recovery after stem cell transplantation: reduction of host neuronal cell death, induction of host brain plasticity with restoration and strengthening of synaptic activity, attenuation of inflammation, increase of neovascularization, and recruitment of endogenous neuronal progenitor cells into the injured brain area (5,20). In order to achieve “bystander” neuroprotective effects, secretion of growth factors and cytokines by the stem cells, or by the recipient’s neuronal cells stimulated by the stem cells, should be considered. Indeed, the search for such key molecules has recently been actively conducted. Yet, the exact molecular mechanisms responsible for the “bystander” contribution of the stem cell in neuronal protection have not been fully explored.
The purposes of this study were to test the hypotheses that stem cell-mediated neuronal protection after hypoxia can be demonstrated in an in vitro co-culture system, and that stem cell co-culture demonstrates significant alterations in protein secretion compared to the control group. This study was designed as a preliminary survey of the levels of protein secretion in the stem cell co-cultured group, in order to guide further studies that will test specific hypotheses relevant to protein regulation and the mechanism of stem cell-mediated neuronal protection. An in vitro experimental method was chosen because of the simplicity of the cells and systems involved in the process, in order to facilitate isolation of potential target proteins for further examination.
MATERIALS AND METHODS
Stem Cells
Rat umbilical cord matrix (RUCM) derived stem cells were used in this study. RUCM cells were isolated from the umbilical cords of female Sprague Dawley®™ Outbred rats (Rattus norvegicus, Harlan Laboratories, Somerville, NJ) at 16-day gestation (20,24). This non-immortalized colonogenic cell line was a kind gift from Kathy E. Mitchell, PhD. RUCM cells were shown to carry stem cell characteristics by localization studies of the embryonic transcription factor Oct-4 (octamer-binding transcription factor 4) and stem cell factor c-kit (20). Indeed, the sub-cultured population (P24 – P30) continued to demonstrate Oct-4 as well as c-kit under the stem cell defined medium, which are composed of Dulbecco’s Modified Eagle’s Medium (Invitrogen, Carlsbad, CA) and MCDB-201 medium(Sigma-Aldrich, Saint Louis, MO) supplemented with 10 μg/ml insulin-5.5 μg/ml transferrin- 6.7 ng/ml selenium (Invitrogen), 0.15% lipid-rich bovine serum albumin (Albumax; Invitrogen), 0.1 nM dexamethasone (Sigma-Aldrich), 10 μM ascorbic acid-2-phosphate (Sigma-Aldrich), 0.01% penicillin/streptomycin (Thermo-Fisher, Suwanee, GA), 2% fetalbovine serum (BD Biosciences, San Jose, CA),10 ng/ml recombinant human epidermal growth factor, and 10 ng/ml rat platelet-derived growth factor BB (R&D Systems, Minneapolis, MN).
The cells maintained stem cell characteristics in a neuronal cell cultured medium (Neurobasal™ Medium: Cat#21103; 2% B27 Serum Free Supplement: Cat. #17504; 8 mM Glutamax™: Cat. #35050; all from Invitrogen) without serum for four days, demonstrating Oct-4 and c-kit immunoactivities.
For re-oxygenation of the neuronal cells after hypoxic stress, P26 sub-cultured RUCM cells were used. They were maintained for three days prior to the seeding in the stem cell defined medium as described above. Immediately prior to seeding, these stem cells were detached from the dish with trypsin (TrypLE™ Express, #12604-013, Invitrogen), the enzyme was blocked with the stem cell defined medium, and was then washed with neuronal cultured medium three times, spun down at 200 × g for 1 minute with maximum speed of 1,100 rpm at 20 °C to remove the element of the stem cell defined medium.
Primary Fetal Neuronal Cells
Primary rat fetal hippocampal neuronal cells were isolated from commercially available, freshly micro-dissected, 18-day gestational fetal rat hippocampi with mixed sexes (NeuroPure™ Primary E18 Rat Hippocampal Cells, Genlantis, Gene Therapy Systems, San Diego, CA) with an established protocol per the company’s specification. Briefly, the hippocampal tissue was pretreated with NeuroPapain Enzyme (2 mg/mL of NeuroPrep™ Medium, Genlantis) for 30 minutes at 30 °C. Following incubation, the cells were spun down at 200 × g for 1 minute with maximum speed of 1,100 rpm at 20 °C and the NeuroPapain solution was removed. The tissue was then mechanically separated with a P-1000 pipette with a sterile 1-mL plastic tip in 1 mL of shipping medium until all the cells were dispersed. The cells were spun down at 200 × g for 1 minute with maximum speed of 1,100 rpm at 20 °C and the supernatant was discarded. The isolated cells were then seeded into a 12-well cultured dish (Costar®, Corning, New York, NY) after poly-D-lysine (Sigma P6407) coating (concentration of the poly-D-lysine with 50 μg/ml) at a density of 104 cells/1.1 cm2 well with 1.0 mL of the neuronal cell culture medium at 37 °C with 21% oxygen and 5% carbon dioxide with 95% humidity. On the third day of the isolation and seeding, neuronal cells appeared to be morphologically differentiated. The neuronal characteristics and purity (> 99%) of the cultured neuronal cells were maintained up to 14 days after isolation and seeding.
Experimental Protocol
Primary neurons freshly isolated from fetal rat hippocampi were seeded as described above and maintained in a 12-well culture dish with 1 mL of the neuronal culture medium in the original culture condition. On the fourth day of culturing, the neurons underwent hypoxia (≤1.0% oxygen) with 5% carbon dioxide and 95% N2 for 96 hours in a modular incubator chamber (MIC-101, Billups-Rothenberg, Del Mar, CA) at 37 °C. The duration of hypoxia was determined based on the results from pilot experiments, in which various hypoxia durations were tested to produce sufficient amount of apoptosis in order to quantify the degree of protection from the stem cells. After the hypoxic period, the neuronal cells were returned to the original normoxic culture condition at 37 °C with 21% oxygen and 5% carbon dioxide with 95% humidity, at which point the study group received the P26 sub-cultured RUCM cells (1 × 104 cells) suspended in 200 μL fresh neuronal culture medium, and the control group received 200 μL fresh neuronal culture medium only (Fig. 1). To prevent accidental removal of neurons from the culture, the neuronal culture medium was not changed once the experiments had begun.
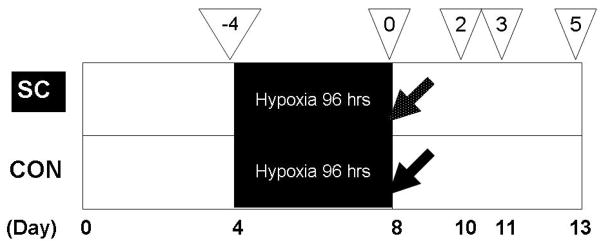
Fig. 1.
Experimental schedule. The dates in the bottom of the figure (Day 0 – 13) indicate the days after isolation and seeding of primary rat fetal neurons from the hippocampus. The numbers in the reversed triangle indicate the days of re-oxygenation after hypoxic stress. Neuronal cell count and neuronal cell apoptosis were examined: 1) immediately prior to hypoxic stress (four days before re-oxygenation), 2) on the second day of re-oxygenation, 3) on the third day of re-oxygenation, and 4) on the fifth day of re-oxygenation. Protein array analysis was performed on the third day of re-oxygenation. The white-speckled arrow indicates resuscitation with rat umbilical cord matrix derived stem cells, while the black arrow indicates resuscitation with culture media only. SC, stem cell group; CON, control group.
Neuronal Cell Count
Neuronal cells were counted in the 12-well culture dish (three independent wells per time point) under a light microscope (Olympus IXY1). With a 100 × visual field, nine predetermined areas per well were selected and photographed. In a blind fashion, the photographed images were analyzed and the neuronal cells were identified by their typical morphology and counted manually. The number of neuronal cells per field was averaged to yield a cell number with standard deviation.
Neuronal Cell Apoptosis
Neuronal cell apoptosis was measured by Annexin V staining [Fluorescein isothiocyanate (FITC) Annexin V Apoptosis Detection Kit I, BD Pharmingen™, Franklin Lakes, NJ]. In order to evaluate apoptosis, the cell culture dish was washed once with 1 mL of phosphate buffered saline solution, added to a mixture of 100 μL of Annexin V Binding Buffer (no. 51-66121E, BD Pharmingen™) and 10 μL of FITC Annexin V (no. 51-65874X, BD Pharmingen™) per manufacturer’s protocol with minor modifications. The dish was placed on a tilting shaker with 60 tilts/min for 15 minutes. Apoptotic cells (Annexin positive) were counted within one hour of the staining’s completion in the 12-well culture dish (three independent wells per time point). The total neuronal cell number was also counted in the same field under a light microscope, and a ratio of apoptotic neuronal cells was then calculated.
Measurement of Protein Concentrations in the Culture Media
Culture media from the co-culture group and the control group on the third day of re-oxygenation were used to determine the expression levels of 90 rat proteins using the RayBio® Biotin Label-based Rat Antibody Array 1 (Cat#:AAR-BLG-1-4: RayBiotech, Norcross, GA). The following were included among the 90 proteins: tissue inhibitor of metalloproteinases-1 (TIMP-1), TIMP-2, TIMP-3, ciliary neurotrophic factor receptor-α (CNTF-R-α), activin A, fractalkine, chemokine (C-C motif) receptor 4 (CCR4), macrophage inflammatory protein-2 (MIP-2), protein kinase, AMP-activated, alpha 1 catalytic subunit (AMPK α1), and tumor necrosis factor receptor superfamily, member 19 (TROY). A complete list of the 90 proteins measured is given (Table 1). The manufacturer-suggested protocol was followed. The culture media were collected, centrifuged at 1,000 × g for 10 minutes, aliquoted, and dialyzed with a dialysis tube. Labeling reagent was added and incubated at room temperature for 30 minutes, and 3 μL of the stop solution was added to the above reaction solution and immediately dialyzed. The biotin-labeled sample was then placed onto a glass chip and incubated at room temperature. The membranes were blocked by incubation with the blocking buffer at room temperature for 30 minutes. After blocking buffer was decanted from each well, 400 μL of each sample was added into appropriate wells. The glass arrays with the culture media sample were incubated at room temperature for two hours. Fluorescent dye-conjugated streptavidin (cy3 equivalent) was then added to each subarray and incubated at room temperature for two hours in the dark. After washing with wash buffers I and II, the glass arrays were dried completely in a sterile environment. The signals were visualized with a laser scanner using the cy3 channel. Relative protein levels were obtained by subtracting the background staining and normalizing to the positive controls on the same membrane. Each assay reading was performed in triplicate, and the mean value was calculated. The fold-change of the co-culture group over the control group was reported for each protein array.
Table 1.
The list of the 90 proteins measured.
| # | Name of the proteins |
|---|---|
| 1 | Activin A |
| 2 | ACTH |
| 3 | ADFP |
| 4 | Adiponectin/Acrp30 |
| 5 | AMPK alpha 1 |
| 6 | B7-1/CD80 |
| 7 | BDNF |
| 8 | beta-Catenin |
| 9 | basic-FGF |
| 10 | beta-NGF |
| 11 | CCR4 |
| 12 | CD106 |
| 13 | CINC-2 alpha/beta |
| 14 | CINC-3 |
| 15 | CNTF |
| 16 | CNTF R alpha |
| 17 | CSK |
| 18 | CXCR4 |
| 19 | EGFR |
| 20 | EG-VEGF/PK1 |
| 21 | E-Selectin |
| 22 | FADD |
| 23 | Fas Ligand/TNFSF6 |
| 24 | Fas/TNFRSF6 |
| 25 | FGF-BP |
| 26 | Follostatin-like -1(FSL1) |
| 27 | Fractalkine |
| 28 | GFR alpha-1 |
| 29 | GFR alpha-2 |
| 30 | GM-CSF |
| 31 | Growth Hormone |
| 32 | Growth Hormone R |
| 33 | Hepassocin |
| 34 | ICAM-1/CD54 |
| 35 | ICK |
| 36 | IDE (Insulin Degrading Enzyme) |
| 37 | IFN-gamma |
| 38 | IL-1 alpha |
| 39 | IL-1 beta |
| 40 | IL-1 R6/IL-1 R rp2 |
| 41 | IL-2 |
| 42 | IL-3 |
| 43 | IL-4 |
| 44 | IL-5 |
| 45 | IL-6 |
| 46 | IL-10 |
| 47 | IL-12/IL-23 p40 |
| 48 | IL-13 |
| 49 | Integrin alpha M beta 2 |
| 50 | Inuslin |
| 51 | IP-10 |
| 52 | Leptin (OB) |
| 53 | LIX |
| 54 | L-Selectin/CD62L |
| 55 | MCP-1 |
| 56 | MDC |
| 57 | MIF |
| 58 | MIP-1 alpha |
| 59 | MIP-2 |
| 60 | MIP-3 alpha |
| 61 | MMP-13 |
| 62 | MMP-2 |
| 63 | MMP-8 |
| 64 | MuSK |
| 65 | Neuropilin-2 |
| 66 | NGFR |
| 67 | Orexin A |
| 68 | Osteopontin/SPP1 |
| 69 | PDGF-AA |
| 70 | Prolactin R |
| 71 | RAGE |
| 72 | RALT/MIG-6 |
| 73 | RELM beta |
| 74 | Resistin |
| 75 | TAL1A |
| 76 | TGF-beta1 |
| 77 | TGF-beta2 |
| 78 | TGF-beta3 |
| 79 | Thrombospondin |
| 80 | TIE-2 |
| 81 | TIMP-1 |
| 82 | TIMP-2 |
| 83 | TIMP-3 |
| 84 | TLR4 |
| 85 | TNF-alpha |
| 86 | TRAIL |
| 87 | TROY |
| 88 | Ubiquitin |
| 89 | VEGF |
| 90 | VEGF-C |
Data Presentation and Statistical Analysis
The data were presented as mean ± s.d. The data between the study group and the control group were compared using unpaired Student’s t test when appropriate. The result was considered statistically significant when the p value was less than 0.05. Statistical analysis was performed using the SPSS/PC+ Advanced Statistics Package, version 13.0 (SPSS, Chicago, IL).
Each value of the protein array assay is presented as a fold change of the co-cultured group from the control group. An equal to or greater than 2.0 fold change was considered a meaningful increase and an equal or less than 0.5 fold change was considered a meaningful decrease in production of the proteins.
RESULTS
Neuronal Cell Count
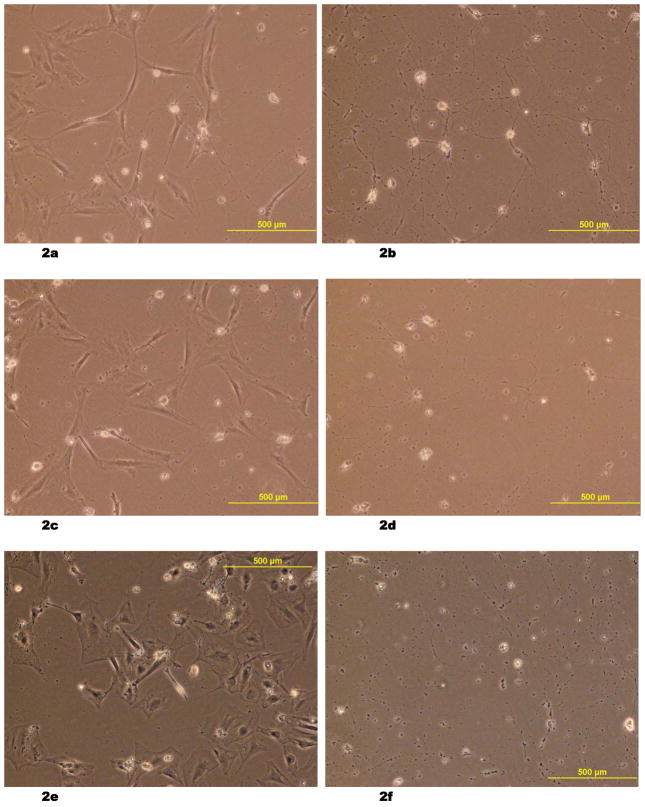
The differences in size and morphology between the neuronal cells and RUCM cells were distinctive throughout the experiment period (Fig. 2a – 2f). Neuronal cell count was higher in the co-cultured group than in the control group (7.3 ± 0.5 cells/100 × field vs. 3.7 ± 0.9 cells/100 × field, p = 0.004 on the third day of recovery; 4.9 ± 1.2 vs. 2.2 ± 0.3, p = 0.02 on the fifth day of recovery) (Fig. 3).
Fig. 2.
(2a – 2f) Light microscopic images of fetal rat neuronal cells, which underwent 96 hours of hypoxic stress and re-oxygenation with resuscitation by co-culturing with rat umbilical cord matrix derived stem cells on Day 2 (2a), Day 3 (2c) and Day 5 (2e). The size and morphology of the neuronal cells are distinctive from the larger stem cells. The control groups after the hypoxic stress and re-oxygenation were also shown on Day 2 (2b), Day 3 (2d), and Day 5 (2f).
Fig. 3.
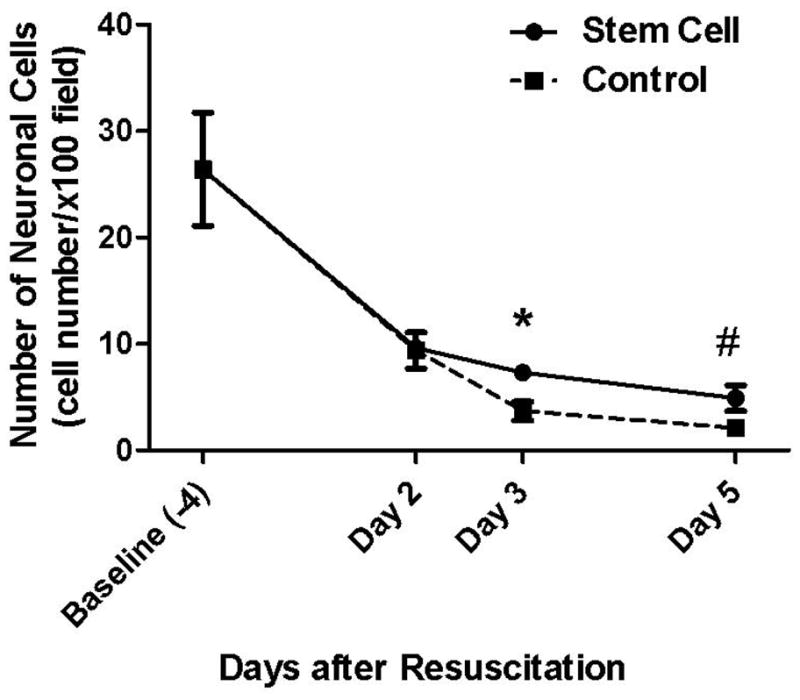
Neuronal cell count. Neuronal cell count was significantly higher in the stem cell co-cultured group than in the control group on Day 3 (* p = 0.004) and on Day 5 (# p = 0.02) of recovery.
Neuronal Cell Apoptosis
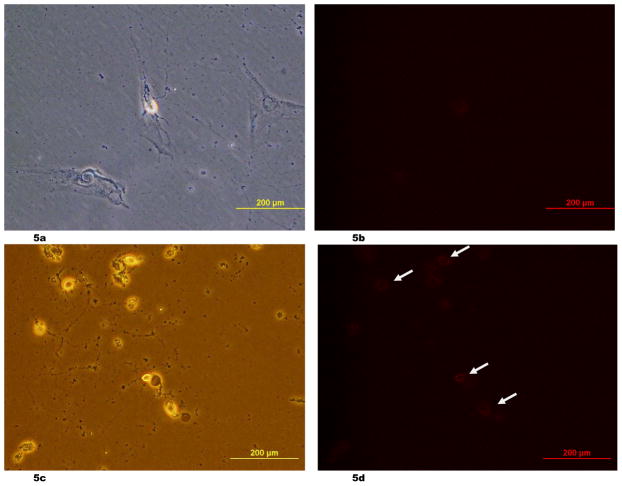
The percentage of apoptotic neurons was significantly lower in the stem-cell-treated group than in the control group, being 44.5 ± 1.6 % and 71.0 ± 4.2 % (p = 0.0005), respectively, on the fifth day of recovery (Fig. 4). Representative images of Annexin V staining were shown (Fig. 5a – 5d).
Fig. 4.
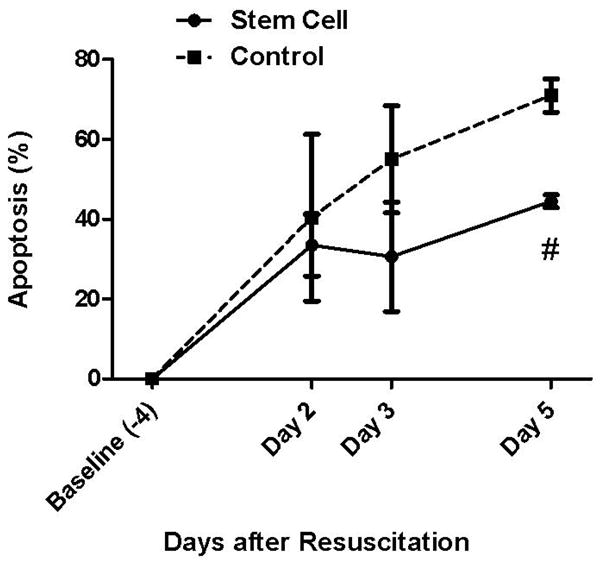
Neuronal cell apoptosis. The percentage of apoptotic neurons was significantly lower in the stem cell co-cultured group than in the control group on Day 5 (# p = 0.0005) of recovery.
Fig. 5.
(5a – 5d) Representative images of Annexin V staining of the fetal rat neuronal cells. The light microscopic image of the neuronal cells with co-cultured stem cells (the co-cultured group) on Day 3 of resuscitation (5a) and its Annexin V staining (5b), which was negative, were shown. The light microscopic image of the neuronal cells of the control group on Day 3 of resuscitation (5c) and its Annexin V staining (5d), which was positive in the neuronal cells (arrows), were also presented. The light microscopic image of the figure 5c was taken with the green fluorescent light.
Protein Array Analysis
Compared to the control group, the culture media from the stem cell co-culture group showed increased secretion in TIMP-1 by 5.61 fold, TIMP-2 by 4.88 fold, CNTF-R-α by 3.42 fold, activin A by 2.20 fold, fractalkine by 2.04 fold, and CCR4 by 2.02 fold. On the other hand, decreased secretion was found in MIP-2 by 0.30 fold, AMPK α1 by 0.43 fold, TROY by 0.48 fold, and TIMP-3 by 0.50 fold (Table 2).
Table 2.
Protein array analysis
| PROTEIN | FOLD CHANGE |
|---|---|
| TIMP-1 | 5.61 |
| TIMP-2 | 4.88 |
| CNTF R alpha | 3.42 |
| Activin A | 2.2 |
| Fractalkine | 2.04 |
| CCR4 | 2.01 |
| MIP-3 alpha | 1.97 |
| CD106 | 1.97 |
| ICK | 1.88 |
| TGF-beta2 | 1.88 |
| TAL1A | 1.82 |
| IL-4 | 1.77 |
| CINC-3 | 1.68 |
| CINC-2 alpha/beta | 1.59 |
| GM-CSF | 1.57 |
| MCP-1 | 1.53 |
| GFR alpha-2 | 1.49 |
| IL-6 | 1.44 |
| beta-NGF | 1.43 |
| VEGF | 1.42 |
| Neuropilin-2 | 1.42 |
| CXCR4 | 1.41 |
| IL-1 beta | 1.4 |
| IL-10 | 1.37 |
| MuSK | 1.36 |
| CNTF | 1.36 |
| LIX | 1.32 |
| E-Selectin | 1.32 |
| IL-1 R6/IL-1 R rp2 | 1.31 |
| B7-1/CD80 | 1.3 |
| ACTH | 1.3 |
| MIP-1 alpha | 1.3 |
| IP-10 | 1.28 |
| Growth Hormone | 1.24 |
| GFR alpha-1 | 1.24 |
| MMP-13 | 1.22 |
| IL-1 alpha | 1.21 |
| PDGF-AA | 1.19 |
| RALT/MIG-6 | 1.15 |
| Adiponectin/Acrp30 | 1.14 |
| RAGE | 1.14 |
| BDNF | 1.14 |
| IL-13 | 1.12 |
| Prolactin R | 1.11 |
| Inuslin | 1.09 |
| MMP-2 | 1.09 |
| MMP-8 | 1.09 |
| basic-FGF | 1.08 |
| Orexin A | 1.03 |
| EG-VEGF/PK1 | 1.03 |
| Resistin | 1.03 |
| Fas/TNFRSF6 | 1.02 |
| Fas Ligand/TNFSF6 | 1 |
| Follostatin-like -1(FSL1) | 0.97 |
| ADFP | 0.97 |
| L-Selectin/CD62L | 0.96 |
| Leptin (OB) | 0.94 |
| RELM beta | 0.94 |
| Growth Hormone R | 0.93 |
| ICAM-1/CD54 | 0.93 |
| FGF-BP | 0.91 |
| IL-5 | 0.9 |
| Integrin alpha M beta 2 | 0.81 |
| TLR4 | 0.71 |
| IL-12/IL-23 p40 | 0.7 |
| TGF-beta3 | 0.68 |
| Ubiquitin | 0.58 |
| TIE-2 | 0.53 |
| TIMP-3 | 0.50 |
| TROY | 0.48 |
| AMPK alpha 1 | 0.43 |
| MIP-2 | 0.30 |
TGF-β1, beta-Catenin, CSK, EGFR, FADD, Hepassocin, IDE (Insulin Degrading Enzyme), IFN-gamma, IL-2, IL-3, MDC, MIF, NGFR, Osteopontin/SPP1, Thrombospondin, TNF-alpha, TRAIL, and VEGF-C were excluded from the final analysis, because the intensity of these proteins failed to reach the threshold-value calculated from the blank controls.
DISCUSSION
This study demonstrated that direct co-culturing of RUCM derived stem cells with primary rat fetal hippocampal neuronal cells after 96 hours of hypoxia decreased neuronal cell apoptosis and neuronal cell death. The study also showed that TIMP-1, TIMP-2, CNTF-R-α, activin A, fractalkine, and CCR4 were present in the stem cell co-cultured media with equal to or more than a 2-fold change compared to those in the control media, while MIP-2, AMPK α1, TROY, and TIMP-3 were shown to have equal to or less than a 0.5 fold change.
Several recent studies have directly explored the mechanisms of stem cell-mediated neuronal protection using an in vitro model. In various culture conditions of human umbilical cord blood (HUCB) cells, Newman et al. found consistent expression of cytokines such as Interleukin-8 (IL-8), monocyte chemoattractant protein-1 (MCP-1), and IL-1α (25). Crigler and associates found that human mesenchymal stem cells expressed brain-derived neurotrophic factor (BDNF) and β-nerve growth factor (β-NGF), but not neurotrophin-3 (NT-3) or NT-4 (12). Hau and colleagues examined the human umbilical cord blood mononuclear cell (HUCB-MNC)-mediated neuronal protective effect on SH-SY5Y cells after 48 hours of hypoxic culture condition (18). They demonstrated significant reduction of apoptosis of neuronal type cells derived from SH-SY5Y cells with post-hypoxic direct co-culture with HUCB-MNC. The cytokine profile of culture media using a cytometric bead array did not show altered production of neuroprotective cytokines [granulocyte colony-stimulating factor (G-CSF), vascular endothelial growth factor (VEGF)] in HUCB-MNC-co-culture vs. mono-culture. It showed, however, a significantly increased production of chemokines (CCL3, CCL4, CCL5, CXCL10) and of inflammatory cytokines (CXCL-8), but not of IL-1β or IL-6 (18). Unlike our study, an immortalized neuronal cell line (SH-SY5Y) was used, and the number of proteins examined was very limited. The same group further demonstrated that a stem cell depleted fraction (CD133 negative) of HUCB-MNC also had the capability to reduce apoptosis in differentiated SH-SY5Y cells after hypoxic condition, and to increase production of CCL3 and G-CSF (29). Most recently, Arien-Zakay and colleagues demonstrated that HUCB-MNC may exert neuroprotection over hypoxic-injured PC12 cells through reduction of oxidant(s) and secretion of neurotrophic factors, including nerve growth factor, vascular endothelial factor, and basic fibroblast growth factor by paracrine interaction between the insulted PC12 and the HUCB-MNC (2). Tate and colleagues have found that human mesenchymal stromal cells (MSCs) and MSC-derived SB623 cells rescued neural cells following an in vitro stroke model (34). Among 30 cytokines tested, they identified 11 neurotrophic factors secreted by MSCs and/or SB623 cells with a fold-change of 1.5 or greater than the control group without any cells, including bone morphogenetic protein (BMP)-4, Dickkopf (DKK)-1, fibroblast growth factor (FGF)-7, heparin binding-epidermal growth factor like growth factor (HB-EGF), hepatocyte growth factor (HGF), IL-6, IL-8, MCP-1, matrix metalloproteinase (MMP)-1, platelet-derived growth factor (PDGF-AA), and VEGF. All the proteins except DKK-1 have been reported to have potential beneficial mechanisms (34).
The new pattern of various protein production and secretion found in our study merits further discussion. The observed changes in the amount of TIMPs in the medium are particularly intriguing. In our protein array analysis, TIMP-1 and TIMP-2 showed 5.61-fold and 4.88-fold increases, respectively. On the other hand, TIMP-3 demonstrated a 0.50 fold change in the stem cell co-cultured media. The TIMP family is a series of glycoproteins expressed by different tissues of several organisms. These proteins are a natural inhibitor of MMPs, a group of peptidases involved in degradation of the extracellular matrix. Among the four members of the TIMP family, TIMP-1, -2 and -3 have recently been found to play a significant role in survival of various cell types, including neuronal cells, mainly through inhibitory function of MMPs. Chen et al. demonstrated this concept in a rat model, in which a broad spectrum synthetic MMP inhibitor, GM6001, provided both acute and long-term neuroprotection in the developing brain by reducing degradation of tight junction proteins, preserving blood-brain barrier (BBB) integrity, and ameliorating brain edema after neonatal hypoxic-ischemic injury (10). Furthermore, TIMP-1 appears to promote cell proliferation in a wide range of cell types and may have anti-apoptotic function and a neuroprotective role due to direct inhibition of MMP-9. Fujimoto and colleagues showed that in TIMP-1(−/−) mice, MMP-9 protein expression and gelatinolytic activity were significantly more augmented after cerebral ischemia than those in wild type mice and were accompanied by exacerbated BBB disruption, neuronal apoptosis, and ischemic injury (15). Magnouni and associates reported the first compelling in vivo evidence that gene transfer of TIMP-1 and TIMP-2 through adenoviral vector-mediated delivery to the mouse brain was effective in producing increased levels of TIMPs, which effectively reduced the extent of neuronal damage induced by transient global ischemia in mice (22). Similarly, Baker and coworkers demonstrated that a combination of intra-arterial injection of autologus bone marrow cells and over-expression of TIMP-1 and TIMP-2 with gene delivery in the rat brain showed the largest benefit both in reduction of infarct size and in recovery of motor function compared to the individual interventions in a rat model of transient cerebral ischemia (4). Indeed, in co-culture media of human umbilical cord mesenchymal stem cells with rat pancreatic islet-like cell clusters, Chao and colleagues have found more than a twofold increase in levels of several cytokines including TIMP-1 and TIMP-2 in co-culture medium (8).
Recent research further indicated that TIMPs also protect neuronal function directly, without relying on inhibition of MMPs (3,13). Tan and colleagues demonstrated that within hours of applying TIMP-1 in recombinant form or by adenovirus-mediated gene transfer, neurons were highly protected against excitotoxic injury. However, a synthetic MMP inhibitor failed to reproduce such neuroprotective effects of TIMP-1 over-expression in vitro (33). The study suggested that non-MMP-related biological functions of TIMP-1 might be responsible, at least in part, for the observed neuroprotection by TIMP-1 in the model (33). TIMP-2 was also reported to induce both cell-cycle arrest and neurite outgrowth, which was independent of MMP inhibitory activity (27).
TIMP-3, on the other hand, appears to induce neuronal damage. Although some contradictory reports exist (28), TIMP-3 was reported to be associated with neuronal apoptosis after ischemia (38). Recently, TIMP-3 gene deletion was shown to prevent Fas-mediated cell death in focal ischemia (39).
Taken together, growing evidence suggests that augmentation of TIMP-1 and TIMP-2 and inhibition of TIMP-3 could be beneficial for neuronal protection. Our study is the first to suggest such a change in the pattern of TIMPs’ production and/or secretion in stem cell co-culture experiments.
In this study, several proteins showed significant increases in the measurable amount in the medium. CNTF R-α (3.42 fold change) binds ciliary neurotophilic factor, and both the receptor and its cognate ligand support neuronal survival (14). Activin (2.2 fold change), which enhances follicle stimulating hormone biosynthesis and secretion, has also been found to be active in cell proliferation, wound repair, and neuronal protection (11,32). Fractalkine (2.04 fold change), or chemokine (CX3C motif) ligand 1, is a unique chemokine that functions as an adhesion molecule as well as a chemoattractant and is expressed on endothelial cells activated by proinflammatory cytokines (35). An increasing body of literature suggests that the chemokine system, including fractalkine, plays a crucial role in brain development and function (1). CCR4 (2.02 fold change) is a receptor for CC chemokines, which induce the migration of monocytes and other cell types, such as dendritic cells. Chemokines and chemokine receptors are widely expressed in the nervous system, where they play roles in the regulation of stem cell migration, axonal path finding, and neurotransmission. Chemokine signaling is also of key importance in the regulation of neuroinflammatory responses (40). All of these proteins are potential candidate proteins responsible for stem cell-mediated neuronal protection.
Several proteins also demonstrated a decreased amount in our study. MIP-2 (0.30 fold change), or chemokine (CXC motif) ligand 2 (CXCL2), is a cytokine belonging to the CXC chemokine family. MIP-2 increases after injury, and neurons appear to be the source of this expression (30). After controlled cortical impact injury in adult rats, rapid up-regulation of MIP-2 was observed after injury (36). The reduction of MIP-2 expression in stem cell co-culture media may relate to reduced injury process of the neuronal cells. AMPK alpha 1 (0.43 fold change) is the catalytic subunit of AMPK. AMPK serves as a metabolic sensor and it regulates the activity of the master regulator of mitochondria, peroxisome proliferator-activated receptor-gamma coactivator 1 alpha, to maintain metabolic homeostasis. It protects cells from stresses that cause ATP depletion by switching off ATP-consuming biosynthetic pathways (7,17). The reduction of AMPK in the stem cell co-culture group may indicate decreased stress level in the neuronal cells. Lastly, TROY (0.48 fold change), or TAJ, is a tumor necrosis factor receptor superfamily, and is broadly expressed in postnatal and adult neurons. TROY blocks neurite outgrowth in the presence of myelin inhibitors by binding to Nogo-66 receptor 1 (26,31). The reduction may indicate less inhibition of neurite outgrowth. In short, all of the above proteins merit further investigation.
It is important to point out that this study is limited by the survey nature of the assessment in protein production and serves as a hypothesis-generating mechanism rather than a definitive identification of the potential proteins involved in stem cell-mediated neuronal protection. Furthermore, although we have identified several proteins with equal to or more than 2-fold change, or equal to or less than 0.5 fold change, in the stem cell co-cultured media, it was beyond the scope of this study to prove that protein changes are directly related to neuronal protection. Additional studies are required to demonstrate the link; for example, one could compare the use of conditioned culture media that is either augmented with a recombinant form of one of the targeted proteins or depleted of the protein using its specific antibody. Another limitation of this study is that only 90 known rat proteins were able to be studied, due to a limited number of currently available protein arrays. Additional study, for example using two-dimensional difference gel electrophoresis (23,41), is needed to fully explore altered protein expression in the stem cell co-cultured group. It should also be noted that the protein levels were measured in the co-culture condition; therefore, a distinction could not be made in this study as to whether the changes of the secretion of the proteins were due solely to the stem cells or to the interaction between the stem cells and the neurons. Future studies with co-culture of non-stem cells or without hypoxia are required to determine whether hypoxia condition is necessary to induce the detected changes and to further differentiate the contributions from stem cells. Lastly, we chose Annexin V staining to evaluate the number of apoptotic neuronal cells in order to rule out any contamination of the result by the inclusion of apoptosis of co-cultured stem cells (18,29). Combined with the cell counting we performed, these two methods provide independent measures for neuronal cell injury and death.
In conclusion, co-culturing of stem cells with primary fetal rat neurons in vitro decreased neuronal cell death after hypoxic stress. Multiple proteins were found to have significant changes in their concentrations in the culture medium after co-culturing with stem cells. These results suggest that stem cells possess the ability to provide cell-mediated neuronal protection, in which an extracellular signaling mechanism could be involved.
Acknowledgments
The research was supported by a Departmental Seed Grant, Department of Anesthesiology, University of Pittsburgh School of Medicine (to T.S.), by Society of Cardiovascular Anesthesiologists/International Anesthesia Research Society Starter Grant (to T.S.), and in part by a grant to Y.X. from the National Institutes of Health (R01NS36124-10). The authors thank Shannon M. Barnes, MS, and Sandra C. Hirsch, MBA, for their editorial assistance with the manuscript.
Footnotes
Presented in part at 104th Annual Meeting of the American Society of Anesthesiologists, New Orleans, LA, USA. October 17-21, 2009.
Neither author has any conflict of interest.
References
- 1.Adler MW, Rogers TJ. Are chemokines the third major system in the brain? J Leukoc Biol. 2005;78:1204–1209. doi: 10.1189/jlb.0405222. [DOI] [PubMed] [Google Scholar]
- 2.Arien-Zakay H, Lecht S, Bercu MM, Tabakman R, Kohen R, Galski H, Nagler A, Lazarovici P. Neuroprotection by cord blood neural progenitors involves antioxidants, neurotrophic and angiogenic factors. Exp Neurol. 2009;216:83–94. doi: 10.1016/j.expneurol.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Baker AH, Edwards DR, Murphy G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci. 2002;115(Pt 19):3719–3727. doi: 10.1242/jcs.00063. [DOI] [PubMed] [Google Scholar]
- 4.Baker AH, Sica V, Work LM, Williams-Ignarro S, de Nigris F, Lerman LO, Casamassimi A, Lanza A, Schiano C, Rienzo M, Ignarro LJ, Napoli C. Brain protection using autologous bone marrow cell, metalloproteinase inhibitors, and metabolic treatment in cerebral ischemia. Proc Natl Acad Sci USA. 2007;104:3597–3602. doi: 10.1073/pnas.0611112104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bliss T, Guzman R, Daadi M, Steinberg GK. Cell transplantation therapy for stroke. Stroke. 2007;38(2 Suppl):817–826. doi: 10.1161/01.STR.0000247888.25985.62. [DOI] [PubMed] [Google Scholar]
- 6.Borlongan CV, Hadman M, Sanberg CD, Sanberg PR. Central nervous system entry of peripherally injected umbilical cord blood cells is not required for neuroprotection in stroke. Stroke. 2004;35:2385–2389. doi: 10.1161/01.STR.0000141680.49960.d7. [DOI] [PubMed] [Google Scholar]
- 7.Cantó C, Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol. 2009;20:98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chao KC, Chao KF, Chen CF, Liu SH. A novel human stem cell coculture system that maintains the survival and function of culture islet-like cell clusters. Cell Transplant. 2008;17:657–664. doi: 10.3727/096368908786092801. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, Sanchez-Ramos J, Chopp M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001;32:2682–2688. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- 10.Chen W, Hartman R, Ayer R, Marcantonio S, Kamper J, Tang J, Zhang JH. Matrix metalloproteinases inhibition provides neuroprotection against hypoxia-ischemia in the developing brain. J Neurochem. 2009;111:726–736. doi: 10.1111/j.1471-4159.2009.06362.x. [DOI] [PubMed] [Google Scholar]
- 11.Chen YG, Wang Q, Lin SL, Chang CD, Chuang J, Chung J, Ying SY. Activin signaling and its role in regulation of cell proliferation, apoptosis, and carcinogenesis. Exp Biol Med. 2006;231:534–544. doi: 10.1177/153537020623100507. [DOI] [PubMed] [Google Scholar]
- 12.Crigler L, Robey RC, Asawachaicharn A, Gaupp D, Phinney DG. Human mesenchymal stem cell subpopulations express a variety of neuro-regulatory molecules and promote neuronal cell survival and neuritogenesis. Exp Neurol. 2006;198:54–64. doi: 10.1016/j.expneurol.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 13.Crocker SJ, Pagenstecher A, Campbell IL. The TIMPs tango with MMPs and more in the central nervous system. J Neurosci Res. 2004;75:1–11. doi: 10.1002/jnr.10836. [DOI] [PubMed] [Google Scholar]
- 14.Davis S, Aldrich TH, Valenzuela DM, Wong VV, Furth ME, Squinto SP, Yancopoulos GD. The receptor for ciliary neurotrophic factor. Science. 1991;253:59–63. doi: 10.1126/science.1648265. [DOI] [PubMed] [Google Scholar]
- 15.Fujimoto M, Takagi Y, Aoki T, Hayase M, Marumo T, Gomi M, Nishimura M, Kataoka H, Hashimoto N, Nozaki K. Tissue inhibitor of metalloproteinases protect blood-brain barrier disruption in focal cerebral ischemia. J Cereb Blood Flow Metab. 2008;28:1674–1685. doi: 10.1038/jcbfm.2008.59. [DOI] [PubMed] [Google Scholar]
- 16.Garbuzova-Davis S, Willing AE, Saporta S, Bickford PC, Gemma C, Chen N, Sanberg CD, Klasko SK, Borlongan CV, Sanberg PR. Novel cell therapy approaches for brain repair. Prog Brain Res. 2006;157:207–222. doi: 10.1016/S0079-6123(06)57014-1. [DOI] [PubMed] [Google Scholar]
- 17.Hardie DG. Roles of the AMP-activated/SNF1 protein kinase family in the response to cellular stress. Biochem Soc Symp. 1999;64:13–27. [PubMed] [Google Scholar]
- 18.Hau S, Reich DM, Scholz M, Naumann W, Emmrich F, Kamprad M, Boltze J. Evidence for neuroprotective properties of human umbilical cord blood cells after neuronal hypoxia in vitro. BMC Neurosci. 2008;9:30. doi: 10.1186/1471-2202-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirko AC, Dallasen R, Jomura S, Xu Y. Modulation of inflammatory responses after global ischemia by transplanted umbilical cord matrix stem cells. Stem Cells. 2008;26:2893–2901. doi: 10.1634/stemcells.2008-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jomura S, Uy M, Mitchell K, Dallasen R, Bode CJ, Xu Y. Potential treatment of cerebral global ischemia with Oct-4+ umbilical cord matrix cells. Stem Cells. 2007;25:98–106. doi: 10.1634/stemcells.2006-0055. [DOI] [PubMed] [Google Scholar]
- 21.Li WY, Choi YJ, Lee PH, Huh K, Kang YM, Kim HS, Ahn YH, Lee G, Bang OY. Mesenchymal stem cells for ischemic stroke: changes in effects after ex vivo culturing. Cell Transplant. 2008;17:1045–1059. doi: 10.3727/096368908786991551. [DOI] [PubMed] [Google Scholar]
- 22.Magnoni S, Baker A, Thomson S, Jordan G, George SJ, McColl BW, McCulloch J, Horsburgh K. Neuroprotective effect of adenoviral-mediated gene transfer of TIMP-1 and -2 in ischemic brain injury. Gene Ther. 2007;14:621–625. doi: 10.1038/sj.gt.3302894. [DOI] [PubMed] [Google Scholar]
- 23.Maurer MH, Kuschinsky W. Screening the brain: molecular fingerprints of neural stem cells. Curr Stem Cell Res Ther. 2006;1:65–77. doi: 10.2174/157488806775269142. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell KE, Weiss ML, Mitchell BM, Martin P, Davis D, Morales L, Helwig B, Beerenstrauch M, Abou-Easa K, Hildreth T, Troyer D, Medicetty S. Matrix cells from Wharton’s jelly form neurons and glia. Stem Cells. 2003;21:50–60. doi: 10.1634/stemcells.21-1-50. [DOI] [PubMed] [Google Scholar]
- 25.Newman MB, Willing AE, Manresa JJ, Sanberg CD, Sanberg; PR. Cytokines produced by cultured human umbilical cord blood (HUCB) cells: implications for brain repair. Exp Neurol. 2006;199:201–208. doi: 10.1016/j.expneurol.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Park JB, Yiu G, Kaneko S, Wang J, Chang J, He XL, Garcia KC, He Z. A TNF receptor family member, TROY, is a coreceptor with Nogo receptor in mediating the inhibitory activity of myelin inhibitors. Neuron. 2005;45:345–351. doi: 10.1016/j.neuron.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 27.Pérez-Martínez L, Jaworski DM. Tissue inhibitor of metalloproteinase-2 promotes neuronal differentiation by acting as an anti-mitogenic signal. J Neurosci. 2005;25:4917–4929. doi: 10.1523/JNEUROSCI.5066-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pi R, Yin W, Zheng S, Qiu P, Zhou J, Guo W, Su T, Yan G. Adenoviral mediated transfer of TIMP-3 partially prevents glutamate-induced cell death in primary cultured cortical neurons of the rat. Mol Brain Res. 2004;127:136–139. doi: 10.1016/j.molbrainres.2004.02.026. [DOI] [PubMed] [Google Scholar]
- 29.Reich DM, Hau S, Stahl T, Scholz M, Naumann W, Emmrich F, Boltze J, Kamprad M. Neuronal hypoxia in vitro: investigation of therapeutic principles of HUCB-MNC and CD133+ stem cells. BMC Neurosci. 2008;9:91. doi: 10.1186/1471-2202-9-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rhodes JK, Sharkey J, Andrews PJ. The temporal expression, cellular localization, and inhibition of the chemokines MIP-2 and MCP-1 after traumatic brain injury in the rat. J Neurotrauma. 2009;26:507–525. doi: 10.1089/neu.2008.0686. [DOI] [PubMed] [Google Scholar]
- 31.Shao Z, Browning JL, Lee X, Scott ML, Shulga-Morskaya S, Allaire N, Thill G, Levesque M, Sah D, McCoy JM, Murray B, Jung V, Pepinsky RB, Mi S. TAJ/TROY, an orphan TNF receptor family member, binds Nogo-66 receptor 1 and regulates axonal regeneration. Neuron. 2005;45:353–359. doi: 10.1016/j.neuron.2004.12.050. [DOI] [PubMed] [Google Scholar]
- 32.Sulyok S, Wankell M, Alzheimer C, Werner S. Activin: an important regulator of wound repair, fibrosis, and neuroprotection. Mol Cell Endocrinol. 2004;225:127–132. doi: 10.1016/j.mce.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 33.Tan HK, Heywood D, Ralph GS, Bienemann A, Baker AH, Uney JB. Tissue inhibitor of metalloproteinase 1 inhibits excitotoxic cell death in neurons. Mol Cell Neurosci. 2003;22:98–106. doi: 10.1016/s1044-7431(02)00024-6. [DOI] [PubMed] [Google Scholar]
- 34.Tate CC, Fonck C, McGrogan M, Case CC. Human mesenchymal stromal cells and their derivative, SB623 cells, rescue neural cells via trophic support following in vitro ischemia. Cell Transplant. 2010;19:973–984. doi: 10.3727/096368910X494885. [DOI] [PubMed] [Google Scholar]
- 35.Umehara H, Bloom ET, Okazaki T, Nagano Y, Yoshie O, Imai T. Fractalkine in vascular biology: from basic research to clinical disease. Arterioscler Thromb Vasc Biol. 2004;24:34–40. doi: 10.1161/01.ATV.0000095360.62479.1F. [DOI] [PubMed] [Google Scholar]
- 36.Vallès A, Grijpink-Ongering L, de Bree FM, Tuinstra T, Ronken E. Differential regulation of the CXCR2 chemokine network in rat brain trauma: implications for neuroimmune interactions and neuronal survival. Neurobiol Dis. 2006;22:312–322. doi: 10.1016/j.nbd.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 37.Vendrame M, Gemma C, de Mesquita D, Collier L, Bickford PC, Sanberg CD, Sanberg PR, Pennypacker KR, Willing AE. Anti-inflammatory effects of human cord blood cells in a rat model of stroke. Stem Cells Dev. 2005;14:595–604. doi: 10.1089/scd.2005.14.595. [DOI] [PubMed] [Google Scholar]
- 38.Wallace JA, Alexander S, Estrada EY, Hines C, Cunningham LA, Rosenberg GA. Tissue inhibitor of metalloproteinase-3 is associated with neuronal death in reperfusion injury. J Cereb Blood Flow Metab. 2002;22:1303–1310. doi: 10.1097/01.WCB.0000040943.89393.c1. [DOI] [PubMed] [Google Scholar]
- 39.Wetzel M, Li L, Harms KM, Roitbak T, Ventura PB, Rosenberg GA, Khokha R, Cunningham LA. Tissue inhibitor of metalloproteinases-3 facilitates Fas-mediated neuronal cell death following mild ischemia. Cell Death Differ. 2008;15:143–151. doi: 10.1038/sj.cdd.4402246. [DOI] [PubMed] [Google Scholar]
- 40.White FA, Feldman P, Miller RJ. Chemokine signaling and the management of neuropathic pain. Mol Interv. 2009;9:188–195. doi: 10.1124/mi.9.4.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wittmann-Liebold B, Graack HR, Pohl T. Two-dimensional gel electrophoresis as tool for proteomics studies in combination with protein identification by mass spectrometry. Proteomics. 2006;6:4688–4703. doi: 10.1002/pmic.200500874. [DOI] [PubMed] [Google Scholar]