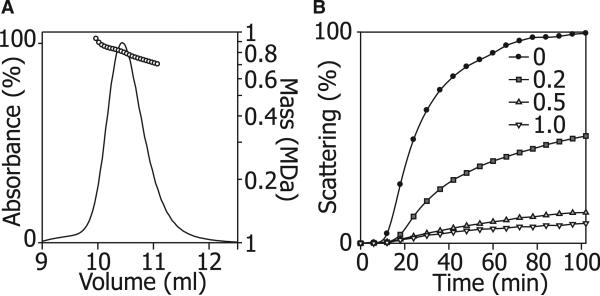
Figure 1. Oligomeric Distribution and Chaperone Activity of ScHSP26.
(A) ScHSP26 examined by SEC-MALS illustrates the heterogeneity of the oligomeric ensemble. The molecular mass decreased across the peak from 950 kDa to 580 kDa and was centered around a maximum population species of 810 kDa, corresponding to an approximately 34 subunit oligomer.
(B) Insulin reduction assay of the chaperone action of ScHSP26. At 25°C, in the absence of ScHSP26, insulin B chain is reduced and exhibits an apparent increase in absorbance at 360 nm due to light scattering over a period of 100 min as a result of aggregation. At a subunit molar ratio of 0.2:1.0 ScHSP26:insulin, a 50% reduction in aggregation was observed due to the chaperone action of ScHSP26. At the higher ratios of 0.5:1.0 and 1.0:1.0, almost complete suppression of aggregation is achieved.