Abstract
Priming of naive CD8+ T cells by pathogens or vaccines generally involves their interaction with Ag-loaded dendritic cells (DCs) in the context of an inflamed lymph node. Lymph node activation fosters DC and T cell encounters and subsequently provides newly primed T cells with nurturing conditions. We dissected these two aspects by infusing in vitro primed CD8+ T cells into naive recipient mice harboring a single activated lymph node and comparing the fate of these T cells with those infused into control recipients. Brief (20 h) in vitro priming empowered the T cells to expand in vivo without further Ag stimulation. This primary response was not affected by the presence or absence of a nonspecifically activated lymph node. In contrast, in vivo antigenic challenge after contraction of the primary response resulted in significantly stronger secondary T cell responses in mice harboring activated lymph nodes, demonstrating that the availability of an activated lymph node supported the generation of T cell memory in an Ag-unrelated manner. The presence of an activated lymph node during the expansion and contraction phase of the primary response did not endow T cells with an instructional program for increased survival or secondary expansion, but primarily served to conserve increased numbers of T cells.
The initiation of T cell responses upon the primary encounter of pathogens involves the delivery of Agand pathogen-associated molecular patterns (PAMPs) to secondary lymphoid organs draining the infected areas of the body. As a result, immunogenic peptide Ags are presented in MHC molecules at the surface of dendritic cells (DCs). Furthermore, the PAMPs trigger a sequence of events that orchestrate effective interaction between Ag-loaded DCs and naive T cells, including the expression of costimulatory molecules at the DC surface and the production of proinflammatory cytokines and chemokines that recruit and arouse a variety of immune cells (1). The latter process causes lymph nodes to develop a state of inflammation and swelling, also referred to as lymph node congestion or activation (2). Chemokines such as CCL9 and CCL21, cytokines including TNF-α and IFN-α/β, and the chemoattractant receptor S1P all play an important role in enhancing the cellularity of the lymph node during an immune response, thereby creating an inflammatory microenvironment supportive of T cell priming (3–8).
The significance of PAMPs, such as TLR ligands, in the induction of T cell immunity has been demonstrated in numerous experimental models, showing that delivery of Ag without PAMPs as immune adjuvants results in T cell tolerance. Although the importance of PAMPs for inducing costimulatory signals through DC activation and for triggering lymph node activation is broadly recognized, and the nature of the DC costimulatory signals has been studied in great detail (9, 10), much less is known about the effects of lymph node activation on the effector and memory phases of the T cell response. In this study, we have focused on the latter aspect by separating DC–T cell engagement and lymph node activation in place and time. Our experimental data reveal that the availability of an inflamed lymph node during the primary response increases the magnitude of the secondary response through the conservation of larger numbers of T cells.
Materials and Methods
Mice
C57BL/6 jico (B6) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). OT-I TCR Tg RAG-1–/– mice were obained from W.R. Heath (The Walter and Eliza Hall Institute of Medical Research, Victoria, Australia) and were bred in the animal facility of Leiden University Medical Center. The experiments were approved by the animal experimental committee of Leiden University Medical Center.
Cell culture
All in vitro cell cultures and assays were performed in IMDM (Invitrogen Life Technologies, Rockville, MD) supplemented with 8% v/v FBS (Greiner, Wemmel, Belgium), 50 μM 2-ME, 2 mM glutamine, 100 IU/ml penicillin (complete medium).
D1 cell line, a long-term growth factor-dependent immature splenic DC line derived from B6 mice, was provided by P. Ricciardi-Castagnoli (University of Milano-Bicocca, Milan, Italy) and cultured as described (11). Bone marrow-derived DCs were isolated from C57BL/6 jico femurs and cultured for 10 d in the same manner as D1 cells before activation.
The engineered APC cell line MEC.B7.SigOVA (SAMBOK) was generated as described (12). The mouse embryonic fibroblast cell line MEC-1 was transfected with CD80 and the minigene SigOVA, which encodes the OVA257–264 (SIINFEKL) peptide directed to the endoplasmatic reticulum, leading to efficient loading of the peptide onto MHC class I molecules Kb. For in vitro priming, engineered APCs were cultured at 70,000 cells per well in 24-well plates overnight. The next day, nonadherent cells and cell debris were removed by washing the wells twice. OT-I cells were then added to the wells (0.5 × 106 cells per well) in 2 ml of medium, and plates were centrifuged for 1 min at 1000 rpm to initiate cell contacts. After 20 h of coculture the nonadherent OT-I cells were gently harvested, washed, and adoptively transferred into recipient mice.
Lymph node activation
D1 cells were cultured in D1 medium, maturation was induced by adding 6 μg/ml LPS (Escherichia coli-derived, serotype O26:B6; Sigma-Aldrich, St. Louis, MO) 24 h before injection. Cells were harvested with EDTA, washed three times with PBS, and 2 × 106 cells in 30 μl PBS were injected into the right hind leg footpad of mice.
In vivo challenge
Fifty micrograms of synthetic short OVA peptide (SIINFEKL) was injected with 15 μg CpG 1826 (synthesized in the Leiden Institute of Chemistry) in 30 μl PBS into the right hind leg footpad of mice.
Flow cytometry
All Abs were purchased at BD Biosciences (San Diego, CA) and eBioscience (San Diego, CA). Intracellular cytokine staining was performed with the Cytofix/Cytoperm kit (BD Biosciences) according to the manufacturer's instructions.
In vitro proliferation assay
Lymph nodes from mice were isolated from recipient mice, and single cells suspensions were generated by mincing through cell strainers. Subsequently, cells were stained with CD8 and CD45.1 mAbs, and OT-I cells were purified by flow cytometric sorting. Equal amounts of cells were cocultured with 100,000 irradiated spleen cells and 1 μg/ml SIINFEKL or medium control. Thirty-six hours later, cells were pulsed with 1 μC 3H per well and analyzed 24 h later.
Virus infection
Mice were infected with 5 × 104 PFUs influenza A/WSN/33 (WSN)-OVAI (13) through intratracheal inoculation. After 5 d, mice were sacrificed, and lungs were isolated in TRizol (Life Technologies) and homogenized. RNA was extracted and purified using the Qiagen (Valencia, CA) RNeasy kit, cDNA was synthesized using 7.5 μg RNA of each sample, and 12.5% cDNA was used in the RT-PCR reaction, in triplicate, and correlated to actin expression (primer/probe; Applied Biosystems, Foster City, CA). cDNA synthesis and RT-PCR were performed using primer sequences as described before (14)
Results
Activated lymph nodes provide increased storage for Ag-experienced T cells
Lymph node activation is an intrinsic aspect of T cell activation by infectious pathogens and vaccines. We separated the T cell priming event from lymph node activation in space and time by exploiting an experimental model consisting of TCR-transgenic OT-I CD8+ T cells and engineered APCs expressing high levels of the cognate OVA-derived peptide epitope and the costimulatory ligand CD80. Prior work had demonstrated that a 20-h in vitro encounter with these APCs empowered naive OT-I cells to vigorously proliferate in vivo upon transfer to naive syngeneic recipient (15). Furthermore, these T cells were shown to develop into long-lived memory cells, capable of clonal expansion and protective effector function in response to secondary Ag encounter (16). Importantly, progression of the 20 h primed OT-I T cells through primary expansion, contraction, and memory phases required neither in vivo exposure to Ag nor the context of an inflamed, congested lymph node. This model therefore offered a unique opportunity to compare the in vivo behavior of primed CD8+ T cells in the absence versus presence of a nonspecifically activated lymph node.
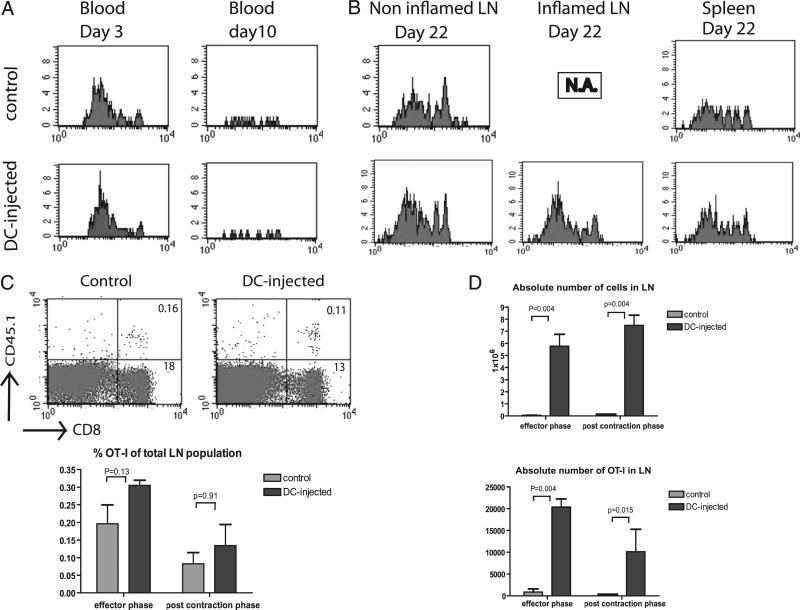
Activation of a single, popliteal lymph node in recipient mice was induced by footpad injection of activated syngeneic DCs (D1 cells or isolated bone marrow DCs) that were not loaded with Ag. Work by others has shown that this results in inflammation of the targeted lymph node within 1 d (8). The use of DCs not loaded with OVA Ag enabled us to separate the impact of Ag exposure and lymph node congestion in time and space. Comparison of the behavior of 20 h ex vivo primed OT-I cells upon injection into DC-injected versus control mice revealed few differences in the kinetics and magnitude of the primary response. In both cases, the percentage of circulating OT-I cells peaked at day 3 after transfer, followed by a rapid contraction caused by the absence of cognate Ag (Fig. 1A). Three weeks after transfer, at a time when OT-I numbers in the blood were below detection, memory T cells could still be detected in the spleen and lymph nodes (Fig. 1B). As observed in the primary phase, percentages of memory OT-I cells differed neither between DC-injected and control mice (Fig. 1C). However, the absolute total cell count was increased in the inflamed lymph nodes compared with contralateral lymph nodes in DC-injected mice and lymph nodes in control mice. Consequently, the absolute numbers of OT-I cells were amplified in these lymph nodes, both at the peak of the effector phase and during the postcontraction phase of the primary response (Fig. 1D). For inflamed lymph nodes, the total cell count was even larger in the postcontraction phase (day 22) than during the effector phase, in line with the notion that the size of the inflamed lymph nodes gradually increases and remains fully enlarged over at least 3 wk (data not shown). Our data suggest that the primary role of an inflamed, enlarged lymph node is to provide an expandable storage reservoir for memory T cells.
FIGURE 1.
Kinetics and magnitude of the primary in vivo response by 20 h in vitro primed OT-I cells. CFSE-labeled naive CD45.1 OT-I cells were cultured during 20 h with engineered APCs expressing OVA Ag and CD80, after which T cells were separated from the APCs and injected into Ag-naive B6 mice (1 × 106 cells per mice) as described previously (15). Recipient CD45.2 mice either did not receive prior treatment or were injected into the footpad of the right hind leg with 2 × 106 cells in vitro, LPS-activated DCs (D1 cells), not loaded with Ag, 24 h prior to T cell infusion. Blood samples were isolated at days 3 and 10 after the start of T cell activation. At day 22, mice were sacrificed, and spleen and lymph nodes were isolated. The panels represent subsequent experiments with similar outcome. OT-I cells were gated by CD8 and CD45.1 A, Representative examples of groups of four mice (A, B) of proliferative response (CFSE) and relative OT-I cell counts in blood of DC-injected and control mice. B, Accumulation of memory OT-I cells during the postcontraction phase in popliteal lymph nodes and spleens of DC-injected and control mice. Right-hand lymph nodes of DC-injected mice were inflamed. Contralateral, left-hand lymph nodes from DC-injected mice served as internal control. N.A., not applicable. Three independent experiments were performed (A, B) C, Percentage of OT-I cells, in relation to total cell counts, in congested, right-hand lymph nodes of DC-injected mice and the same lymph nodes from control mice in the postcontraction phase. Comparison revealed no difference in both effector and postcontraction phase. D, Absolute total cell count and OT-I T cell count in congested, right-hand lymph nodes of DC-injected mice and the same lymph nodes from control mice. Mann-Whitney U test revealed significant differences in total and OT-I numbers in the effector phase as well as in the memory phase: five mice per group, two experiments pooled. Four independent experiments performed in total (C, D).
Memory T cell storage is important for the magnitude of the secondary T cell response
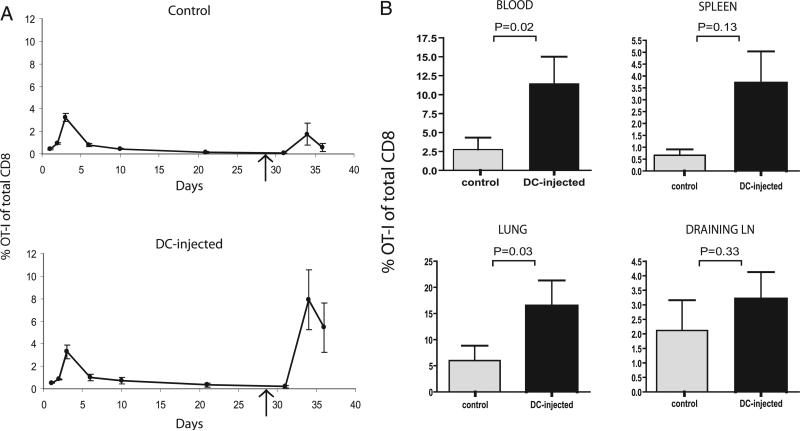
Our initial observations prompted us to test how accumulation of greater numbers of memory T cells in a single inflamed lymph node would affect the systemic CD8+ T cell response upon secondary Ag encounter. We delivered a secondary antigenic challenge to the in vitro primed OT-I cells through injection of OVA peptide and CpG ODN into the footpad of the right hind leg, the same footpad into which mice with inflamed lymph nodes received their initial DC injection. In line with the greater number of memory T cells available in these LNs in DC-injected mice, secondary responses in these mice were stronger than in control mice (Fig 2A, 2B). Moreover, the secondary responses in DC-injected mice were stronger than as compared with the primary responses recorded in these same mice, which is reminiscent of a textbook example of the relative magnitude of primary and secondary T cell responses. Differences between DC-injected and control mice were most prominent in the blood and lung (Fig. 2B), illustrating that secondary challenge of the DC-injected mice resulted in increased frequencies of effector T cells capable of leaving the lymphoid organs and migrating to potential target tissues. Differences in OT-I T cell frequencies were the least prominent in the draining lymph nodes, in line with this migratory behavior of effector T cells and the increased overall cellularity of these lymph nodes (Fig. 1D). Similar results were obtained in mice with an activated lymph node induced by injecting bone marrow-derived DCs (data not shown).
FIGURE 2.
The presence of inflamed LNs during primary response increases the magnitude of the secondary T cell response. Primary responses of ex vivo primed OT-I cells were followed over time in the blood in mice that had received a footpad injection of LPS-activated DCs, not loaded with Ag, and in control mice. At day 28, after contraction of the primary response, mice were challenged with 50 μg synthetic OVA peptide (SIINFEKL) comprising the OT-I T cell epitope in combination with 15 μg CpG. This Ag challenge was delivered into the footpad of the right hind leg, at the same site of DC injection. A, Kinetics of OT-I in blood in control and DC-injected mice, with a mean of three mice per group with SEM. Arrow indicates time of Ag challenge. Representative of three experiments B, Mean values for percentages of OT-I cells in different compartments around peak of secondary response (day 8 after secondary challenge) for 14 mice per group. Mann-Whitney U test revealed statistically significant differences between groups in blood and lungs (p = 0.02; p = 0.03, respectively), but not in spleens and draining LNs (p = 0.13; p = 0.33, respectively). Two independent experiments were performed.
Interestingly, the secondary responses in control mice did not exceed the magnitude of the primary responses (Fig. 2A). Our experiments therefore reveal that the availability of an inflamed lymph node during the primary response is important for the generation of an enhanced secondary T cell response. Because secondary Ag challenge in our experiments involved codelivery of CpG, causing efficient activation of the draining lymph nodes in both DC-injected and control mice, we deem it unlikely that the already inflamed status of these lymph nodes in DC-injected mice is a key determinant in facilitating the secondary response of the T cells. The most likely explanation for the stronger secondary response in DC-injected mice is therefore the greater number of CD8+ memory T cells conserved after contraction of the primary response elicited from the inflamed lymph nodes.
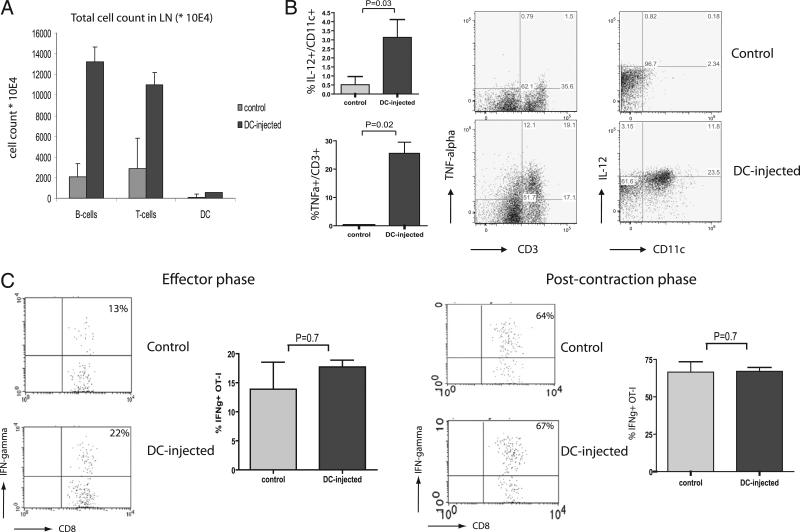
To further analyze the conditions in inflamed lymph nodes in DC-injected mice, we performed footpad injection of CFSE-labeled DCs. As shown in Fig. 3A, this action results in a rapid increase, within 1 d, of the absolute numbers of various types of cells, including T cells, B cells, and CD11c+ DCs. The increase in CD11c+ cell numbers cannot be attributed by the injected DCs, because CFSE-high cells were found to constitute less than 0.5% of the CD11c+ population (data not shown). The host-derived cells recruited to the inflamed lymph node displayed an activated state, as illustrated by the increased frequencies of IL-12–producing CD11c+ cells and TNF-α–producing CD3+ cells (Fig. 3B). There the findings strengthened the notion, based on experiments shown in Fig.1C and 1D, that the injection of activated DCs triggers an overall accumulation of APCs and lymphocytes rather than a selective accumulation and/or activation of Ag-specific OT-I T cells. Accordingly, we did not find significant differences in the activation status, as determined by IFN-γ production, of ex vivo-primed OT-1 cells between inflamed and resting lymph nodes of DC-injected and control mice, respectively, in neither the effector phase nor the post-contraction phase of the primary response (Fig. 3C). Evaluation of additional markers that were found to be associated with T cell activation and memory cell formation [in particular, CD62L, CD127, CD122, CCR7, and TRAIL (17–19)] also failed to reveal a qualitative difference between the OT-I T cells in resting versus inflamed lymph nodes (Supplemental Fig. 1 and data not shown).
FIGURE 3.
Conditions in the inflamed LN. Cellularity and cytokine production in an inflamed and control LNs were analyzed 24 h after footpad injection of LPS-activated DCs. Single-cell suspensions of LNs were stained for CD19, CD3, and CD11c A, Absolute numbers of cells in inflamed and control LNs. B, Percentage cytokine producing cells in inflamed and control LNs 24 h after footpad injection of DCs. Cytokine production was analyzed by intracellular cytokine staining following PMA-ionomycin activation. In each experiment, four mice per group were used. Mann-Whitney U test revealed statistically significant differences in IL-12p40 and TNF-α production between groups (p = 0.03; p = 0.02, respectively). Two independent experiments were performed. C, IFN-γ production by OT-I CD8+ T cells at days 3 and 26 after the start of T cell activation. Single-cell suspensions of LNs were analyzed by intracellular cytokine staining after 3 h of stimulation with SIINFEKL peptide in vitro. Mann-Whitney U test revealed no significant difference between groups in the effector and postcontraction phase (p = 0.7 in both phases). In groups of three mice, three independent experiments were performed.
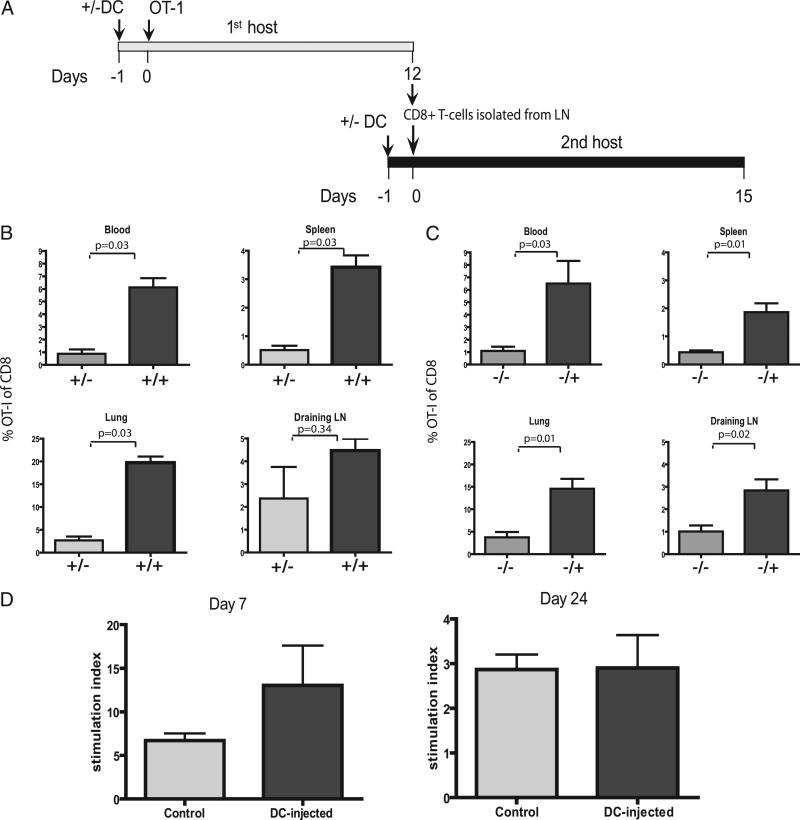
The possibility of a qualitative rather than a mere quantitative difference between OT-I cells in DC-injected and control recipient mice was further investigated by harvesting OT-I memory T cells from mice with inflamed lymph nodes in the early postcontraction phase (12 d after infusion) and transferring these (in identical numbers) to either DC-injected or control recipients (Fig. 4A). T cells were allowed to rest for another 15 d in new recipients, after which they encountered a secondary antigenic challenge through footpad injection of OVA-peptide/CpG. Analysis of the secondary in vivo OT-I responses showed that the availability of an inflamed lymph node in the recipient mice was of key importance for a strong, systemic memory T cell response (Fig. 4B). Thus, post-contraction OT-I T cells harvested from inflamed lymph nodes only persisted in greater quantities when transferred into DC-injected recipients, and therefore did not exhibit an intrinsic capacity for survival independent of the availability of an inflamed lymph node. In reciprocal experiments, we found that postcontraction OT-I cells harvested from control mice led to stronger secondary responses in magnitude when infused into DC-injected mice than when infused into control mice (Fig. 4C), supporting the notion that inflamed LN supports memory T cell maintenance and CD8+ memory T cells educated in DC-injected and control mice did not differ intrinsically.
FIGURE 4.
T cells educated in inflamed LNs are not intrinsically changed. T cells recovered from inflamed or control LNs were counted and adoptively transferred in identical numbers (103 OT-I cells per recipient) into new recipients with or without inflamed LNs. Response to a boost vaccination was analyzed in blood, spleen, lung, and draining LNs. A, Schematic cartoon of experimental design. B, Mean values for percentages of OT-I cells educated in mice with inflamed LNs in different compartments around the peak of secondary response (day 8 after secondary challenge): four mice per group. Mann-Whitney U test revealed significant differences for blood, spleen, and lung (p = 0.03 for all compartments), but not draining LNs (p = 0.34) between groups. C, Mean values for percentages of OT-I cells educated in control mice in different compartments around the peak of secondary response (day 8 after secondary challenge): four mice per group. Mann-Whitney U test revealed significant differences for blood, spleen, lung, and draining LNs (p = 0.03; p = 0.01; p = 0.01; p = 0.02, respectively) between groups. Two independent experiments were performed (A, B). T cells recovered from inflamed or control LNs at days 7 and 24 were isolated by flow cytometric sorting and were cocultured with irradiated spleen cells and SIINFEKL peptide in equal amounts (1250 cells per well for day 7, 500 cells per well for day 24). After 36 h, cells were pulsed with tritium and incorporation was measured 24 h later. Stimulation index was calculated as tritium count with peptide divided by tritium count of medium control. D, Stimulation index of OT-I cells recovered on days 7 and 24, respectively. Groups of 5 (day 7) and 10 (day 24) mice. Mann-Whitney U test revealed no significant differences between groups. Mann-Whitney U test revealed no significant differences on days 7 and 24 (p = 0.25; p = 1.0, respectively). One experiment, representative of two.
To determine whether the OT-I T cells from DC-injected mice reacted differently to antigenic stimuli, OT-I T cells cells were recovered, at days 7 and 24 after adoptive transfer from LNs of DC-injected and control mice, and cocultured in vitro with feeders and specific peptide. Proliferation to the specific peptide was analyzed by tritium incorporation. (Fig. 4D) Although a trend toward more proliferation at day 7 in the DC-injected group was observed, this difference was not significant. At day 24, no difference between OT-I cells from DC-injected versus control mice to the antigenic challenge was observed. This finding supports our hypothesis that the amplified response in vivo in DC-injected mice is due to quantitative difference caused by the larger absolute numbers of Ag-experienced T cells in the inflamed lymph node.
Importance of memory T cell conservation by inflamed LNs in antiviral immunity
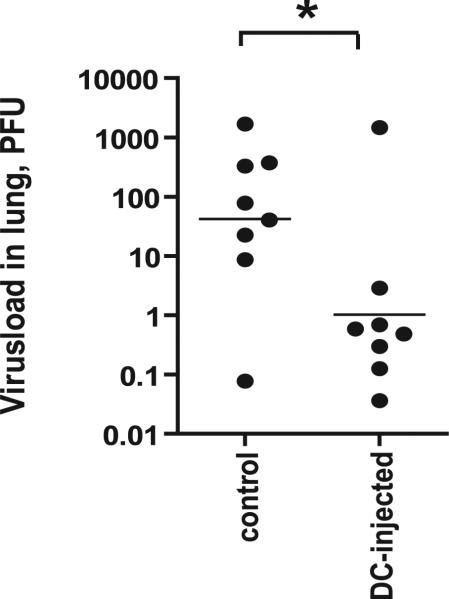
Thus far, our experiments demonstrate that secondary challenge at the site of prior lymph node activation can result in a superior memory T cell response. In additional experiments, we delivered the secondary antigenic challenge, consisting of OVA peptide and CpG ODN, in the ipsilateral footpad. Ag delivery at this site resulted in a secondary T cell response comparable to that elicited by Ag delivery in the foot pad near the inflamed lymph node, showing that the locally conserved memory T cells can be recruited into secondary responses triggered by antigenic delivery at distal sites (data not shown). To further assess the physiologic significance of memory CD8+ T cell conservation in inflamed, local lymph nodes, we infected OT-I recipient, DC-injected, and control mice intratracheally with recombinant influenza virus encoding the OVA Ag (13). As shown in Fig. 5, DC-injected OT-I recipients have a significantly lower amount of virus particles in their lungs. This finding can be explained by the fact that DC-injected recipients develop a memory T cell response superior to that of control OT-I recipients, which is capable of more efficiently controlling the viral infection.
FIGURE 5.
Presence of inflamed LNs during primary response enhances antiviral immunity. A secondary response to an influenza virus containing the SIINFEKL epitope is more vigorous in mice with an inflamed LN, induced by injecting LPS-activated DCs not loaded with Ag into the footpad of mice during the primary response. Mice with an inflamed LN or control mice were adoptively transferred with in vitro activated OT-I T cells. After 35 d, mice were infected with 5 × 104 PFUs influenza virus through intratracheal injection. Five days later, viral load in the lungs was determined by quantitative PCR. Mann-Whitney U test revealed significant reduction of virus RNA in DC-injected mice compared with control mice. p = 0.05; eight mice per group.
Discussion
By separating DC–T cell engagement and lymph node activation in space and time, we demonstrated that the presence or absence of an activated lymph node has no effect on the magnitude and kinetics of the primary response, at least in the absence of cognate Ag, but clearly influences the magnitude of the memory T cell response upon secondary Ag encounter. Furthermore, our data show that the presence or absence of an activated lymph node does not imprint intrinsic differences into the T cells that would result in alternate survival or memory programs. Instead, the activated lymph nodes appear to primarily offer increased storage space for Ag-experienced T cells during the contraction and memory phases of the primary response. This finding offers opportunities for using lymph node inflammation in conditioning patients for adoptive T cell transfer strategies. In addition to the current method of lympho depletion, lymph node inflammation can create a nurturing environment for newly injected T cells. Our findings differ from those emerging from studies that have examined the effects of signals at the DC-T cell interface on the development of T cell memory. For example, the presence of costimulatory signals involving the 4-1BB and OX40 pathways during T cell priming imprints a survival program that promotes development of T cell memory and enhanced secondary T cell responses (20–22). Furthermore, the presence of CD4+ T cells during CD8+ T cell priming was shown to render CD8+ T cells insensitive to TRAIL-mediated death during the secondary response, thereby endowing them with the capacity to mount an enhanced memory T cell response (17, 23). These prior studies and our present work imply that T cell programming during DC–T cell engagement and the availability of enlarged lymph nodes for T cell storage are expected to synergize in the generation of potent T cell memory.
Acknowledgments
This work was supported by a grant from the Netherlands Organization for Scientific Research.
Abbreviations used in this paper
- DC
dendritic cell
- LN
lymph node
- PAMP
pathogen-associated molecular pattern
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Hall JG, Morris B. The immediate effect of antigens on the cell output of a lymph node. Br. J. Exp. Pathol. 1965;46:450–454. [PMC free article] [PubMed] [Google Scholar]
- 3.Chiba K, Yanagawa Y, Masubuchi Y, Kataoka H, Kawaguchi T, Ohtsuki M, Hoshino Y. FTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats. I. FTY720 selectively decreases the number of circulating mature lymphocytes by acceleration of lymphocyte homing. J. Immunol. 1998;160:5037–5044. [PubMed] [Google Scholar]
- 4.Förster R, Schubel A, Breitfeld D, Kremmer E, Renner-Müller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 5.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 6.Shiow LR, Rosen DB, Brdicková N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 7.McLachlan JB, Hart JP, Pizzo SV, Shelburne CP, Staats HF, Gunn MD, Abraham SN. Mast cell-derived tumor necrosis factor induces hypertrophy of draining lymph nodes during infection. Nat. Immunol. 2003;4:1199–1205. doi: 10.1038/ni1005. [DOI] [PubMed] [Google Scholar]
- 8.MartIn-Fontecha A, Sebastiani S, Höpken UE, Uguccioni M, Lipp M, Lanzavecchia A, Sallusto F. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J. Exp. Med. 2003;198:615–621. doi: 10.1084/jem.20030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu. Rev. Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 10.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu. Rev. Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 11.Winzler C, Rovere P, Rescigno M, Granucci F, Penna G, Adorini L, Zimmermann VS, Davoust J, Ricciardi-Castagnoli P. Maturation stages of mouse dendritic cells in growth factor-dependent long-term cultures. J. Exp. Med. 1997;185:317–328. doi: 10.1084/jem.185.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schoenberger SP, Jonges LE, Mooijaart RJ, Hartgers F, Toes RE, Kast WM, Melief CJ, Offringa R. Efficient direct priming of tumor-specific cytotoxic T lymphocyte in vivo by an engineered APC. Cancer Res. 1998;58:3094–3100. [PubMed] [Google Scholar]
- 13.Topham DJ, Castrucci MR, Wingo FS, Belz GT, Doherty PC. The role of antigen in the localization of naive, acutely activated, and memory CD8(+) T cells to the lung during influenza pneumonia. J. Immunol. 2001;167:6983–6990. doi: 10.4049/jimmunol.167.12.6983. [DOI] [PubMed] [Google Scholar]
- 14.Borowski AB, Boesteanu AC, Mueller YM, Carafides C, Topham DJ, Altman JD, Jennings SR, Katsikis PD. Memory CD8+ T cells require CD28 costimulation. J. Immunol. 2007;179:6494–6503. doi: 10.4049/jimmunol.179.10.6494. [DOI] [PubMed] [Google Scholar]
- 15.van Stipdonk MJ, Hardenberg G, Bijker MS, Lemmens EE, Droin NM, Green DR, Schoenberger SP. Dynamic programming of CD8+ T lymphocyte responses. Nat. Immunol. 2003;4:361–365. doi: 10.1038/ni912. [DOI] [PubMed] [Google Scholar]
- 16.van Stipdonk MJB, Sluijter M, Han WGH, Offringa R. Development of CTL memory despite arrested clonal expansion. Eur. J. Immunol. 2008;38:1839–1846. doi: 10.1002/eji.200737974. [DOI] [PubMed] [Google Scholar]
- 17.Janssen EM, Droin NM, Lemmens EE, Pinkoski MJ, Bensinger SJ, Ehst BD, Griffith TS, Green DR, Schoenberger SP. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- 18.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naïve cells. Nat. Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weninger W, Crowley MA, Manjunath N, von Andrian UH. Migratory properties of naive, effector, and memory CD8(+) T cells. J. Exp. Med. 2001;194:953–966. doi: 10.1084/jem.194.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maxwell JR, Weinberg A, Prell RA, Vella AT. Danger and OX40 receptor signaling synergize to enhance memory T cell survival by inhibiting peripheral deletion. J. Immunol. 2000;164:107–112. doi: 10.4049/jimmunol.164.1.107. [DOI] [PubMed] [Google Scholar]
- 21.Gramaglia I, Jember A, Pippig SD, Weinberg AD, Killeen N, Croft M. The OX40 costimulatory receptor determines the development of CD4 memory by regulating primary clonal expansion. J. Immunol. 2000;165:3043–3050. doi: 10.4049/jimmunol.165.6.3043. [DOI] [PubMed] [Google Scholar]
- 22.Bertram EM, Lau P, Watts TH. Temporal segregation of 4-1BB versus CD28-mediated costimulation: 4-1BB ligand influences T cell numbers late in the primary response and regulates the size of the T cell memory response following influenza infection. J. Immunol. 2002;168:3777–3785. doi: 10.4049/jimmunol.168.8.3777. [DOI] [PubMed] [Google Scholar]
- 23.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]