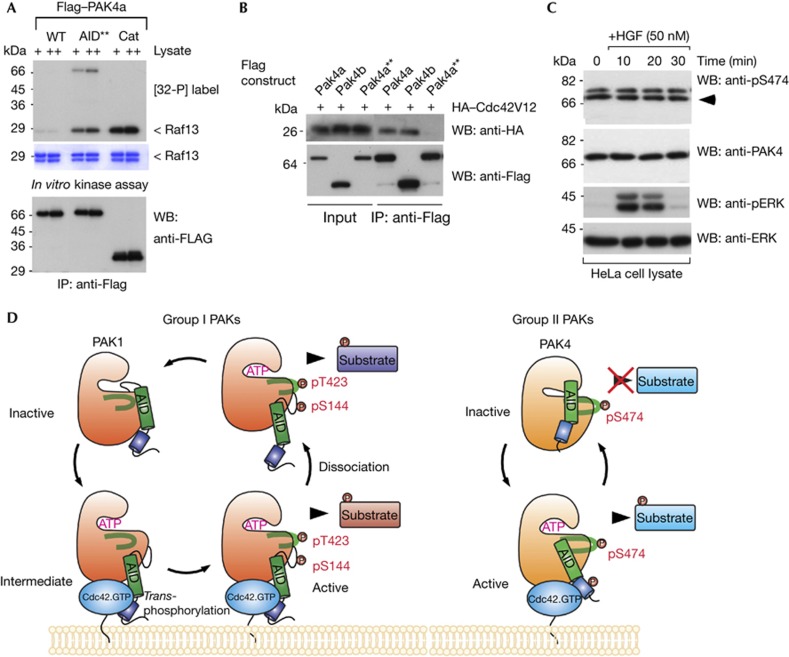
Figure 4.
An auto-inhibitory domain (AID) active mutant has reduced binding to Cdc42. (A) Constructs of wild-type or mutant PAK4a as indicated were expressed in Cos7 cells, immunoprecipitated and assayed for activity in vitro. WT, wild type; AID**, RR48/49AE, T60E; Cat, PAK4(286–591). (B) PAK4a, PAK4b and the active PAK4a–AID** were immunoprecipitated and tested for the presence of coexpressed HA–Cdc42V12. (C) HeLa cells were serum-starved (16 h), treated with HGF and harvested at the times shown. Triton X-100 soluble cell lysates (30 μg/lane) were probed with new antibodies directed to PAK4, and PAK4 pS474, or ERK1/2 and pERK1/2 (Cell Signaling). (D) This model summarizes our findings regarding the alternate activation mechanisms for group I and II PAKs. Cdc42 binding in both cases results in relief from auto-inhibition, but through different underlying mechanisms. In the case of PAK1, this requires new phosphorylation of Ser 144 and Thr 423. For simplicity we do not show the PAK1 as a dimer. In contrast, PAK4 is constitutively phosphorylated on Ser 474, and requires Cdc42.GTP to induce and sustain an active kinase conformation that allows substrate interaction. HA, haemagglutinin; HGF, hepatocyte growth factor; IB, immunoblot; IP, immunoprecipitated; PAK, p21-activated kinase; WB, western blot; WT, wild type.