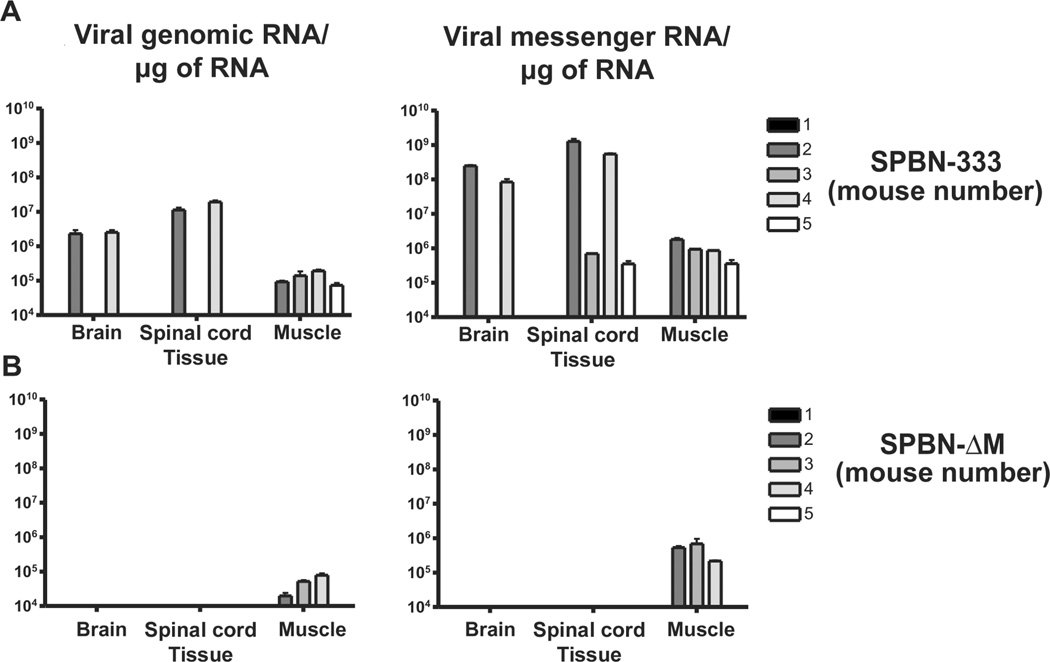
Figure 4.
Safety of the matrix (M) gene–deficient rabies virus (RV) vaccine (SPBN-ΔM). Safety was determined by assessing dissemination to the central nervous system in immunodeficient mice. Groups of 5 immunodeficient (recombination activating gene 2 [RAG2] knockout) mice were inoculated intramuscularly with 105 foci-forming units (FFUs) of either SPBN-333 (A) or SPBN-ΔM (B). Once an animal in either group became moribund due to RV infection, all mice were euthanized. Mice inoculated with the highly attenuated SPBN-333 vector served to define the tissue-collection time points for SPBN-ΔM–immunized mice, since it was not known whether SPBN-ΔM would induce visible signs of RV infection. Total RNA was isolated from muscles, brains, and spinal cords, and viral reverse-transcription polymerase chain reaction and quantitative real-time polymerase chain reaction were used to amplify and quantify viral genomic (left) or messenger (right) RNA from individual samples.