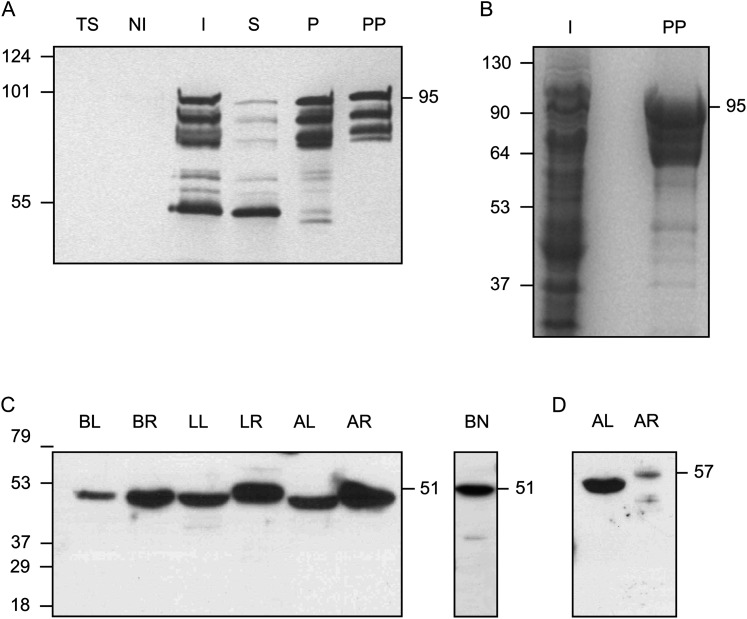
Fig. 1.
Expression and purification of γECS and immunoblots of γ-glutamylcysteine synthetase (γECS) and homoglutathione synthetase (hGSHS) in legumes. (A) Purification of the fusion protein between the γECS from bean and the maltose-binding protein from Escherichia coli. Immunoblot using a monoclonal antibody against the His tag: TS, untransformed E. coli BL21 cells (10 μg protein); NI, non-induced transformed cells (10 μg protein); I, induced transformed cells (10 μg protein); S, supernatant of lysed induced cells (10 μg protein); P, pellet of lysed induced cells (2.5 μg protein); PP, purified recombinant enzyme (0.5 μg protein). (B) SDS gel stained with Coomassie. I, induced transformed cells (50 μg protein); PP, purified recombinant enzyme (10 μg protein). The expected molecular mass of the fusion protein is ∼95 kDa. (C, D) Immunoblots of γECS in several organs of representative legumes (C) and hGSHS in alfalfa (D) (20 μg protein). BL, bean leaves; BR, bean roots; BN, bean nodules; LL, Lotus japonicus leaves; LR, Lotus japonicus roots; AL, alfalfa leaves; AR, alfalfa roots. Detection was by chemiluminescence with the SuperSignal West Pico (A–C) or Femto (D) kits. Molecular masses (kDa) of the protein markers are shown on the left and apparent molecular masses (kDa) of the proteins are given on the right.