Abstract
Three different types of non-photochemical de-excitation of absorbed light energy protect photosystem II of the sun- and desiccation-tolerant moss Rhytidium rugosum against photo-oxidation. The first mechanism, which is light-induced in hydrated thalli, is sensitive to inhibition by dithiothreitol. It is controlled by the protonation of a thylakoid protein. Other mechanisms are activated by desiccation. One of them permits exciton migration towards a far-red band in the antenna pigments where fast thermal deactivation takes place. This mechanism appears to be similar to a mechanism detected before in desiccated lichens. A third mechanism is based on the reversible photo-accumulation of a radical that acts as a quencher of excitation energy in reaction centres of photosystem II. On the basis of absorption changes around 800 nm, the quencher is suggested to be an oxidized chlorophyll. The data show that desiccated moss is better protected against photo-oxidative damage than hydrated moss. Slow drying of moss thalli in the light increases photo-protection more than slow drying in darkness.
Keywords: Chlorophyll fluorescence, energy conservation, energy dissipation, photoprotection, photosystem II, reaction centre
Introduction
After light has been absorbed in the pigment system of photosynthetic organisms, energy conservation is initiated by charge separation and electron transfer reactions within specialized reaction centres (RCs) of the photosynthetic apparatus. Within the RCs of photosystem II (PSII), primary photo-induced electron transfer from an electron donor termed P680 to the electron acceptor pheophytin creates a strong oxidant, P680+, and a reduced pheophytin, Pheo–. The former is capable of oxidizing water. The latter reduces the primary quinone electron acceptor QA within the RC. Further electron transfer reactions, which include photosystem I (PSI), result finally in the reduction of CO2.
Problems arise when either water or electron acceptors, or both, are unavailable and light intensity is high. In that case, light damages the photosynthetic apparatus. The recombination of charges on P680+ and Pheo– activates oxygen (Krieger-Liszkay, 2005; Asada, 2006; Krieger-Liszkay et al., 2008). Singlet oxygen, 1O2, is highly oxidative. Damage by 1O2 or other oxidants formed in strong light can be prevented or minimized if excess light energy is rapidly converted into heat by the action of non-photochemical quenching mechanisms. In hydrated plants, a main mechanism of thermal energy dissipation is inactive in low light. It is activated under excess light. For activation, it requires the presence of the xanthophyll zeaxanthin and the light-dependent protonation of a thylakoid protein (Demmig-Adams, 1990; Niyogi, 1990; Björkman and Demmig-Adams, 1994; Ma et al., 2003; Li et al., 2004; Takizawa et al., 2007). Light intensity controls this energy dissipation mechanism preventing competition with RCs, which remain active for photosynthesis even under strong light.
In desiccated photoautotrophs, persistence of normal RC activity under strong sunlight would cause serious photo-oxidative damage. It would endanger survival. Many mosses (more than 15,000 species in total) and most lichens (more than 13,000 species) are desiccation-tolerant (Lakatos, 2011). Full photo-protection in the desiccated state requires mechanisms of energy dissipation, which are more effective in dissipating light energy thermally than the zeaxanthin-dependent photo-protection, which operates in hydrated photoautotrophs.
Recently, picosecond measurements of fluorescence lifetimes have revealed a new mechanism of photoprotection in desiccated lichens (Veerman et al., 2007; Komura et al., 2010; Miyake et al., 2011). Migration of excitation energy to a far-red emitting pigment protein permits de-excitation of the bulk pool of excited chlorophyll, which is faster in the desiccated than in the hydrated state. Energy transfer with short time constants of 0.31, 23, and 112 ps appeared to drain excitation energy from PSII RCs (Komura et al., 2010; Miyake et al., 2011). This protects PSII RCs against photo-inactivation.
An earlier work with a shade-adapted moss, Rhytidiadelphus squarrosus, observed activation of zeaxanthin-dependent energy dissipation while the moss was hydrated (Heber et al., 2006a). On desiccation of the moss, photo-protective energy dissipation increased. This was attributed to the formation of a radical in PSII RCs, which is capable of quenching excitation energy. It is not yet known whether the energy dissipation mechanism that operates in the moss is related to a mechanism reported for desiccated lichens that exhibits picosecond fluorescence decay (Veerman et al., 2007; Komura et al., 2010; Miyake et al., 2011).
The present study used a closely related desiccation-tolerant moss species, Rhytidium rugosum, which survives full exposure to sunlight in contrast to shade-adapted Rhytidiadelphus squarrosus. The following questions were addressed: (1) Is Rhytidium rugosum, when desiccated, protected also by a mechanism of thermal energy dissipation, which is similar to that shown to prevent photo-oxidative damage to desiccated lichens? (2) What is the role of the radical observed earlier in Rhytidiadelphus squarrosus in desiccated Rhytidium rugosum, and is it stable? (3) Is the recombination of unstable radical pairs such as P680+ and Pheo– non-toxic or potentially toxic by giving rise to the activation of oxygen in desiccated Rhytidium rugosum? (4) Does the effectiveness of photo-protection of hydrated moss differ from that of desiccated moss? To answer these questions, modulated fluorescence and fluorescence lifetimes were measured. Measurements of absorption changes in the far-red served to identify radicals in desiccated moss thalli.
Materials and methods
The moss Rhytidium rugosum (Ehrh.) Kindb., family Rhytidiaceae, was collected either in the dry or the hydrated state from a sun-exposed location on calcareous soil near Leinach, 25 km from Würzburg, Bavaria, Germany. This site served repeatedly as a source of material during different seasons in the years 2006–2010. After collection, hydrated moss was slowly dried in dim light (<4 μmol m−2 s−1 photosynthetically active photon flux density, PPFD) or in complete darkness and stored in darkness at a relative humidity below 65% or a water potential below –70 MPa before being used for experiments. Prolonged dark adaptation (usually 36 or 48 h) of hydrated thalli was intended to decrease zeaxanthin levels. Zeaxanthin is converted to violaxanthin in the dark or in low light (Björkman and Demmig-Adams, 1994).
The yield of chlorophyll fluorescence was measured beyond 700 nm after excitation with 650 nm light in a pulse amplitude modulation fluorometer (model 101, Walz, Effeltrich, Germany) (Schreiber et al., 1986). A modulated measuring beam of low intensity (average PPFD of 0.04 or 2.5 μmol m−2 s−1) served to elicit fluorescence. Halogen lamps (KL 1500, Schott, Mainz, Germany) provided strong short light pulses (usually 1 s) and continuous illumination of white light through heat- and far-red-absorbing filters (Calflex c and DT-Cyan, Balzers, Liechtenstein) and fibre optics. The PPFD of the light pulses was 10,000 μmol m−2 s−1 unless otherwise stated. During prolonged illumination with strong continuous light, the temperature of moss samples was monitored by a thermocouple and regulated by a cooling system. PPFDs were measured by a LI-COR189 quantum sensor (Fa Walz, Effeltrich, Germany).
Experiments with gas mixtures of different CO2 concentrations were performed using a sandwich-type cuvette, which permitted controlled gas flow over hydrated moss thalli. Light-dependent absorption changes were measured in reflection using the pulse amplitude modulation fluorometer in combination with ED800 T emitter/detector units (Walz) equipped with different LEDs. Peak emissions of the LEDs were at 802, 835, 875, and 950 nm.
Fluorescence lifetimes were measured as reported in Komura and Itoh (2009) and Komura et al. (2010). Fluorescence of dry and wet moss thalli was excited by a 430-nm laser pulse. The excitation light was obtained from a Ti:Sapphire laser (Mai Tai; Spectra-Physics, Newport Corporation, Irvine, CA, USA). The frequency-doubled light at 430 nm was generated by a type-I BBO crystal from an 860-nm laser pulse with a pulse duration of 150 fs and a repetition rate of 80 MHz. Fluorescence was focused onto the entrance slit of a 50-cm polychromator and was detected by a streak camera detector (50 cm, Chromex 2501-S, 100 g/mm, Hamamatsu Photonics, Hamamatsu, Japan) as described previously. The streak camera system was operated in the photon-counting mode to give 640 (wavelength) × 480 (time) pixel 2D images for a 636–778 nm fluorescence emission range with 1-nm resolution and 1100- or 5350-ps time range. The signal was accumulated for approximately 0.5–1 h in each measurement or as described elsewhere in detail (Komura and Itoh, 2009; Komura et al., 2010).
The following parameters are described: Fo, minimum yield of modulated fluorescence at the dark-adapted level, indicating that QA of PSII is oxidized; Fs, stationary yield above Fo, indicating that QA is partially reduced; Fm, maximum yield, indicating that QA is fully reduced; Fv, variable fluorescence, calculated as Fm–Fs or Fm–Fo. The value of Non-photochemical fluorescence quenching (NPQ) is calculated as Fm/Fm' – 1 or, after desiccation, as Fm/Fo dessicated – 1 because Fm' is very close to Fo dessicated; then, Fo hydrated/Fo dessicated is a parameter to quantify the extent of competition between functional RCs and dissipation centres to capture excitons.
Glutaraldehyde, dithiothreitol (DTT), and nigericin were obtained from Sigma-Aldrich/Fluka (Seelze, Germany).
Results
Fluorescence emission spectra of hydrated and desiccated thalli
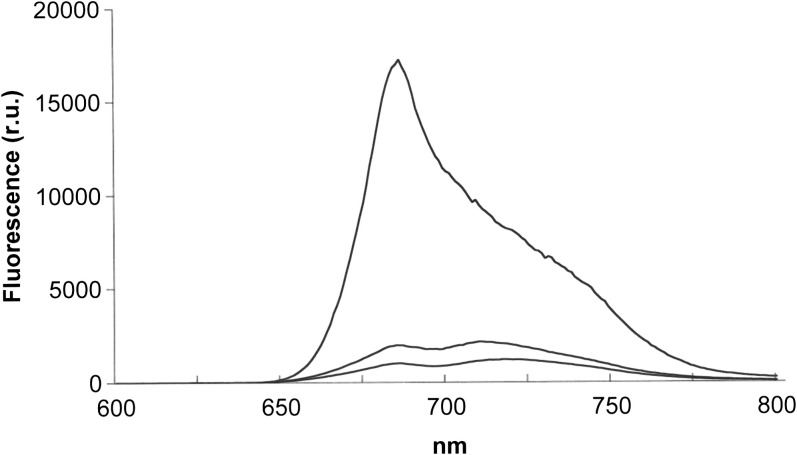
Fig. 1 shows fluorescence emission spectra of Rhytidium rugosum measured at room temperature. Emission around 680 nm originates mainly from PSII. Emission above 700 nm contains contributions of PSI, which is less fluorescent than PSII at room temperature. Desiccation suppressed fluorescence at 680 nm more strongly than above 700 nm. Moss desiccated in darkness had bands with maxima close to 680 and 710 nm (middle spectrum). Desiccation in the light decreased fluorescence more than desiccation in darkness. It shifted the peak of the far-red band to about 720 nm (lower spectrum).
Fig. 1.
Fluorescence emission spectra of hydrated and desiccated thalli of Rhytidium rugosum. Upper, middle, and lower traces represent the spectra of hydrated thalli, dark-adapted desiccated thalli after drying in darkness, and light-adapted thalli after drying in the light (PPFD 600 μmol m−2 s−1), respectively. Excitation wavelength was 480 nm.
The colour of the moss was not changed much by desiccation. Therefore, the large desiccation-induced loss of fluorescence cannot be ascribed to altered light absorption. It can be explained by effective competition between the radiation-less energy dissipation processes induced by desiccation and the fluorescence emission process in PSII.
Fluorescence lifetime measurements of hydrated and desiccated thalli
Fig. 2 shows 2D images of fluorescence decay (wavelength on the X-axis and delay time with respect to the 430-nm excitation laser flash on the Y-axis) of desiccated (A, dry) and hydrated (B, wet) thalli of Rhytidium rugosum measured at room temperature (25 °C). Excitation of fluorescence was at 430 nm. Each photon emitted as fluorescence emission from moss was detected by a charge-coupled device in a streak camera system as reported by Komura and Itoh (2009) and Komura et al. (2010). Each dot on the image indicates the trace of a photon accumulated in the photon-counting mode in the apparatus. As can be seen from the very short tail along the Y-axis of fluorescence at 680 nm of the image, which disappeared at 0.5 ns after the flash excitation, the decay of fluorescence was very fast in the dry thalli at all wavelengths (Fig. 2A). However, within a few minutes after re-wetting the thalli, the Y-axis tail of the fluorescence became longer (Fig. 2B). It should also be noted that the range of emission wavelengths did not change at all, although lifetime changed significantly.
Fig. 2.
Fluorescence decay kinetics of hydrated and desiccated Rhytidium rugosum at room temperature. (A, B) Wavelength-decay time 2D image of fluorescence of desiccated (A) and rehydrated (B) thalli. (C) Fluorescence spectra calculated by integrating photons in whole time domains of the images in A (red, desiccated) and B (blue, rehydrated) with the A spectrum normalized to the highest peak of the B spectrum (red dotted trace). (D) Semi-logarithmic plots of fluorescence decay time courses at 670, 690, 720, and 750 nm, calculated from the images of desiccated (red) and hydrated (blue) thalli.
Fig. 2C compares the fluorescence emission spectra of dry and wet thalli, calculated as the integration of all counts over the measurement times in the two images of Fig. 2A and B. A dotted line also indicates the expanded spectrum of the dry thalli after normalization of the peak heights. As already shown in Fig. 1, the spectrum of dry thalli showed smaller peak height than that of the wet thalli, especially in the 650–700 nm region, indicating the decrease of PSII fluorescence. The smaller integrated intensities in the dry thalli in Fig. 2C, therefore, come mainly from the faster decay of PSII fluorescence after desiccation as shown in Fig. 2A and 2B.
Fig. 2D shows the decay kinetics of fluorescence at different wavelengths with respect to the laser excitation time. The apparent decay times (1/e) at 670, 690, 720, and 750 nm were 487, 596, 354, and 403 ps in the wet thalli (Table 1). They were 105, 137, 113, and 137 ps in the dry thalli. The acceleration ratios at 670 and 690 nm were 4.6 and 4.4, respectively, and larger than those of 3.1 and 2.9 at 720 and 750 nm. The time courses at 750 nm also indicated some rising phase immediately after the excitation. This suggests energy transfer from the shorter wavelength bands in the dry moss. The result indicates acceleration by desiccation of energy migration from PSII antenna (at 670 nm) and the reaction centre (680–690 nm bands) towards the far-red lower energy bands, which dissipate energy as heat and do not transfer energy back to the shorter bands in an uphill process.
Table 1.
Apparent decay time constant (τ1/e) of fluorescence decay at each wavelengths in wet and dry thalli of Rhytidium rugosum
| Fluorescence wavelength (nm) | Wet τ1/e (ps) | Dry τ1/e (ps) | Acceleration ratio (wet/dry) |
| 670 | 487 | 105 | 4.6 |
| 690 | 596 | 137 | 4.4 |
| 720 | 354 | 113 | 3.1 |
| 750 | 403 | 137 | 2.9 |
Each decay time constant was calculated from the time course at each wavelength shown in Fig. 2D.
The result of measurements of fluorescence lifetime also indicates that desiccation of moss accelerates the decay of chlorophyll fluorescence. The effect is similar to that reported recently for desiccated lichens (Veerman et al., 2007; Komura et al., 2010; Miyake et al., 2011). The extent of acceleration in Rhytidium under the present condition was a little smaller compared to that reported for lichens. The results suggest the induction by desiccation of a new energy dissipation pathway in PSII that quenches excitation energy.
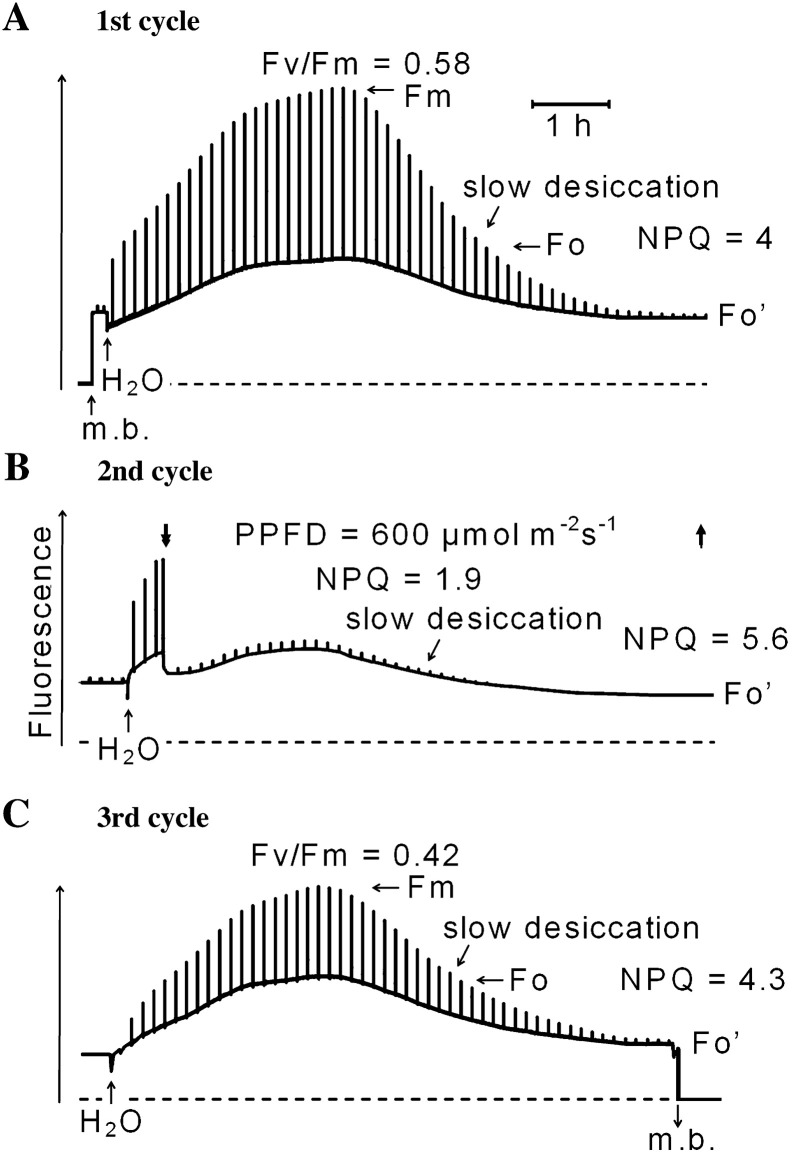
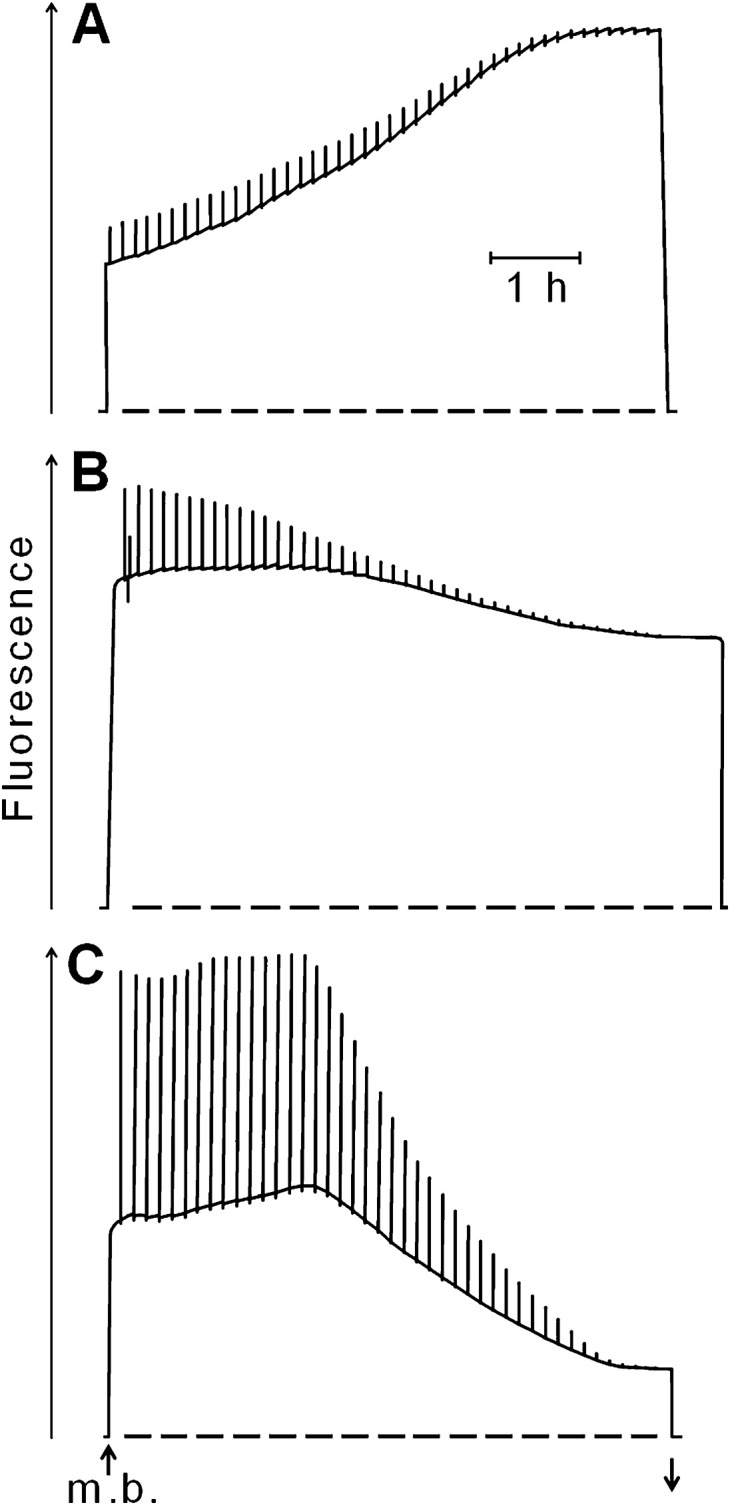
Effects of consecutive hydration/desiccation cycles on modulated chlorophyll fluorescence emission
Fig. 3A–C shows changes of modulated fluorescence of Rhytidium rugosum during three consecutive hydration-desiccation cycles that lasted about 24 h in total. Excitation was done with a PPFD of 0.04 μmoles m−2 s−1 (during the first and third hydration cycles) to establish near-darkness conditions. Strong light pulses (6000 μmol m−2 s−1) of 1 s duration interrupted near-darkness conditions once every 500 s. PPFD was 2 μmoles m−2 s−1 during the second hydration cycle where additional actinic light was given to decrease noise levels. The light pulses served to monitor the maximum level of fluorescence yield (Fm). Before the start of the first cycle, hydrated moss had been dark-adapted for 36 h. It was then dried in darkness to decrease zeaxanthin levels.
Fig. 3.
Modulated chlorophyll fluorescence of Rhytidium rugosum during three consecutive hydration/desiccation cycles as monitored by a pulse amplitude modulation fluorometer. (A) First hydration–dessication cycle: addition of water to desiccated moss increases stationary and pulse-induced fluorescence, and this is followed by fluorescence decline during slow drying. (B) Second hydration–dessication cycle: fluorescence increased initially by addition of water as in A but illumination with a PPFD of 600 μmol m−2 s−1 decreased both stationary and pulse-induced fluorescence, and this is followed by fluorescence decline during slow drying. (C) Third hydration–dessication cycle: fluorescence increased initially as in A but pulse-induced fluorescence responses were smaller than in A owing to illumination in B. For further explanation, see text. Measuring beam (m. b.) was turned on and off as shown by up and down arrows, respectively.
In the first hydration–desiccation cycle in the desiccated moss (Fig. 3A), two strong light pulses elicited small transient fluorescence responses, which indicated some charge separation in the PSII RCs followed by the reduction of the primary quinone electron acceptor QA (Duysens and Sweers, 1963). Fluorescence decreased slightly immediately upon hydration. This is probably caused by the oxidation of QA – (see also Fig. 1B in Heber et al., 2006a for the moss Rhytidiadelphus). Subsequently, fluorescence increased from the minimum level slowly towards a maximum. Pulse-induced fluorescence responses increased strongly towards Fm. The ratio of variable to maximum fluorescence [(Fm–Fo)/Fm = Fv/Fm] is known to be a measure of the quantum efficiency of energy conversion in PSII RCs (Genty et al., 1989). Fv/Fm increased to 0.58 during the first hydration phase (Fig. 3A). This value is lower than maximum values of Fv/Fm (about 0.8), which are reported for unstressed higher plants (Björkman and Demmig, 1987). Fv/Fm ratios of hydrated thalli observed in the field after darkening were variable depending on previous exposure to strong light.
Both the steady-state fluorescence yield and fluorescence responses towards Fm decreased as the moss dried out. This is direct evidence of the activation of photo-protective energy dissipation mechanisms because energy dissipation, fluorescence and photosynthetic reactions are competitive to one another. Non-photochemical fluorescence quenching (NPQ), a conventional measure of the efficiency of thermal energy dissipation, increased from zero to 4 during the first dehydration phase (Fig. 3A) while the moss dried out.
A second hydration cycle increased fluorescence again (Fig. 3B). Illumination at approximately 50% of bright sunlight (600 μmol m−2 s−1) decreased both steady-state fluorescence and pulse-induced fluorescence responses suggesting activation of photo-protective thermal energy dissipation. NPQ increased to 1.9 in the hydrated condition. Desiccation then increased NPQ to 5.6. Strong light pulses given after complete desiccation failed to elicit fluorescence responses. Illumination was turned off before the third hydration phase.
In the third cycle (Fig. 3C), addition of water once again increased steady-state fluorescence. Pulse-induced fluorescence responses increased to a level smaller than that observed in the first hydration phase (Fig. 3A). The peak Fv/Fm value in the third cycle was 0.42 compared to 0.58 during the first hydration. This shows that the stable charge separation in PSII RCs, indicated by the Fv/Fm values, had decreased by more than 25%. This is seen as a consequence of the illumination given during the second hydration phase (Fig. 3B). Apparently, the activation of energy dissipation that produced an NPQ of 1.9 during the preceding illumination period in the second cycle (Fig. 3B) had not been sufficient for full photo-protection of PSII RCs. NPQ returned to 4.3 after the third hydration phase (Fig. 3C).
The ratios of Fohydrated/Fo'desiccated were also calculated to reveal how much faster excitation energy is trapped in active dissipation centres of desiccated thalli than in open RCs of hydrated thalli. Fo/Fo' was 2.3 after the first hydration–dehydration cycle. It became 3.9 under the influence of illumination during the second hydration–dehydration cycle. When illumination was absent during the third hydration–dehydration cycle, Fo/Fo' returned to 2.8.
In a number of additional experiments similar to those shown in Fig. 3, in which hydrated dark-adapted moss was exposed to 600 μmol m−2 s−1 for a few hours, loss of stable charge separation was as large as or larger than that detected in the experiment of Fig. 3. It is therefore concluded that strong illumination of hydrated sun-tolerant moss cannot prevent appreciable photo-damage to PSII RCs. The observations appear to explain the considerable variation in Fv/Fm values of hydrated Rhytidium in the field.
Energy dissipation in hydrated moss: activation by protonation and inhibition by DTT
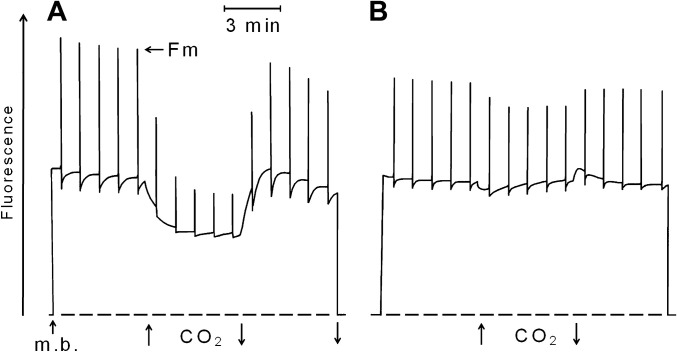
Increased NPQ under strong light during the second hydration cycle in Fig. 3B (NPQ 1.9) suggests involvement of zeaxanthin in energy dissipation (Demmig-Adams, 1990). Zeaxanthin-dependent energy dissipation is known to be controlled by the protonation of the PsbS protein (Li et al., 2004) or a similar thylakoid protein (Benente et al., 2010). In low light or darkness, zeaxanthin is reconverted to violaxanthin. Fig. 4 shows a fluorescence experiment in which hydrated thalli of Rhytidium rugosum, which had been dark adapted for 48 h, received strong light pulses given 1 min apart. In Fig. 4A, replacement of air by 20% CO2 in air decreased not only Fm but also steady-state fluorescence, Fs, that was slightly above Fo. As shown by Bukhov et al. (2001), the potential acid CO2 is capable of replacing light as a source of protons for the activation of energy dissipation in hydrated mosses and lichens provided zeaxanthin is present. Removal of CO2 largely reversed fluorescence quenching. The observation of CO2-dependent quenching in Fig. 4A suggests that thalli of dark-adapted Rhytidium rugosum still contained zeaxanthin.
Fig. 4.
Effect of 20% CO2 in air on the stationary (Fs) and maximum fluorescence level (Fm) of Rhytidium rugosum. (A) Hydrated thallus darkened for 48 h to decrease zeaxanthin levels before measurement. (B). Thallus as in A, but also incubated for 2 h with 5 mM DTT before measurement. Modulated light to support fluorescence at the Fs level had a PPFD of 4 μmol m−2 s−1; the saturating light pulses (1 s) had a PPFD of 10,000 μmol m−2 s−1. Measuring beam (m. b.) was turned on and off and CO2 was increased as shown by arrows.
DTT is known to be an inhibitor of the light-dependent de-epoxidation of violaxanthin to zeaxanthin (Yamamoto and Kamite, 1972). The experiment of Fig. 4B shows that DTT is also an inhibitor of the activation of energy dissipation by protonation. After dark-adapted moss thalli were incubated for 5 h with 5 mM DTT, 20% CO2 failed to decrease steady-state fluorescence. As DTT cannot prevent protonation reactions caused by CO2, it is concluded that inhibition of the activation of energy dissipation by DTT occurs at a step in the signal transduction chain of zeaxanthin-dependent energy dissipation, which is beyond the protonation reaction.
Moss thalli, which had been pre-incubated for 5 h in 5 mM DTT, lost fluorescence during desiccation as much as that in hydrated controls (data not shown). This shows that DTT does not inhibit desiccation-induced energy dissipation. The protonophore nigericin, another inhibitor of zeaxanthin-dependent energy dissipation, was also ineffective to inhibit desiccation-induced energy dissipation (data not shown).
Effects of glutaraldehyde on desiccation-induced energy dissipation
Glutaraldehyde possesses two aldehyde groups that can react with proteins (Coughlan and Schreiber, 1984). In Fig. 5A, fluorescence was measured immediately after hydration of dried moss in 0.2% aqueous solution of glutaraldehyde. Fluorescence increased slowly. It did not decrease while the moss dried. In Fig. 5B, fluorescence was measured after hydrated thalli had been pre-incubated for 1 h with 0.2% aqueous glutaraldehyde. During drying, fluorescence decreased, but much less than that in the control experiment (Fig. 5C), in which dry thalli had been hydrated in water for 1 h before fluorescence was measured.
Fig. 5.
Changes in modulated chlorophyll fluorescence in hydrated Rhytidium rugosum during slow desiccation. (A) Desiccated moss thalli were hydrated in 0.2% aqueous glutaraldehyde shortly before the measuring beam was turned on. (B) Rehydrated thalli were exposed for 1 h to 0.2% glutaraldehyde before the measuring beam was turned on. (C) Control experiment without glutaraldehyde, otherwise as in B. Saturating light pulses were given every 500 s to probe for stable charge separation in PSII RCs. Measuring beam (m. b.) was turned on and off as shown by up and down arrows, respectively.
A comparison of the three experiments of Fig. 5 shows that 0.2% aqueous glutaraldehyde decreased, but did not fully inhibit, pulse-induced charge separation in PSII RCs. Drying caused complete or strong loss of charge separation. Importantly, drying resulted in strong loss of fluorescence only in the control experiment (Fig. 5C) but not in the glutaraldehyde experiments (Fig. 5A and B). The data suggest involvement of glutaraldehyde-sensitive proteins in desiccation-induced energy dissipation.
Desiccation-induced energy dissipation is highly effective in providing phototolerance
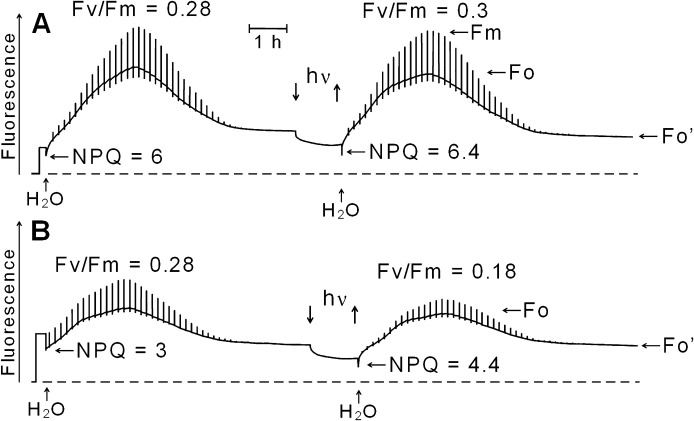
Fig. 6 shows modulated chlorophyll fluorescence during two consecutive hydration/desiccation cycles of Rhytidium rugosum. In Fig. 6A, the moss had been dried slowly immediately after collection in the sun. In Fig. 6B, the moss, collected from the same place, had been dark-adapted in the hydrated state for 36 h. It was then dried in the dark.
Fig. 6.
Modulated chlorophyll fluorescence during two consecutive hydration/desiccation cycles of Rhytidium rugosum interspaced by a 60 min illumination period (PPFD of 10,000 μmol−2 s−1) of the dry moss. (A) Thalli exposed for 4 days to full sunshine during a high-pressure period in early October and dried. (B) Thalli obtained as in A hydrated in near-darkness for 2 days in the laboratory and then dried in darkness. Strong light pulses (1 s) were given every 500 s. For explanation, see text.
Initial NPQ in the dry state was 6 in Fig. 6A. After hydration in near-darkness and subsequent desiccation it was reduced to 3.5 (not shown in Fig. 6A). This is a result of partial relaxation of desiccation-induced energy dissipation during hydration in darkness. The maximum Fv/Fm ratio was 0.28 during hydration. The ratio Fo/Fo', a measure of the suppression of Fo by desiccation, was about 5 before and 3.3 after the hydration. This also reveals relaxation of desiccation-induced energy dissipation. A 60 min exposure of the dry moss to 10,000 μmol m−2 s−1, i.e. to almost eight times maximum sunlight, decreased fluorescence of the desiccated moss. Most of this decrease was not reversed by darkening. A subsequent hydration increased fluorescence to give the maximum Fv/Fm of 0.3. This shows that exposure of the desiccated moss to extremely strong irradiation for 1 h had not damaged the PSII RCs.
The only difference between the experiments in Fig. 6A and B was dark adaptation of the moss used for Fig. 6B. Fo/Fo' was 2.7 in Fig. 6A and 1.9 in Fig. 6B. This reveals loss of photo-protection during the dark adaptation. Extremely strong illumination for 60 min (10,000 μmol m−2 s−1) for 60 min decreased fluorescence of the dry thalli as shown in Fig. 6A. The subsequent hydration increased fluorescence again, but pulse-induced fluorescence responses were now decreased compared to those in the first hydration/desiccation cycle. Maximum Fv/Fm was 0.18, which is lower than the value of 0.28 observed before strong illumination of the dry moss. The result confirms that dark-adaptation of hydrated moss had increased the sensitivity to photo-inactivation after drying.
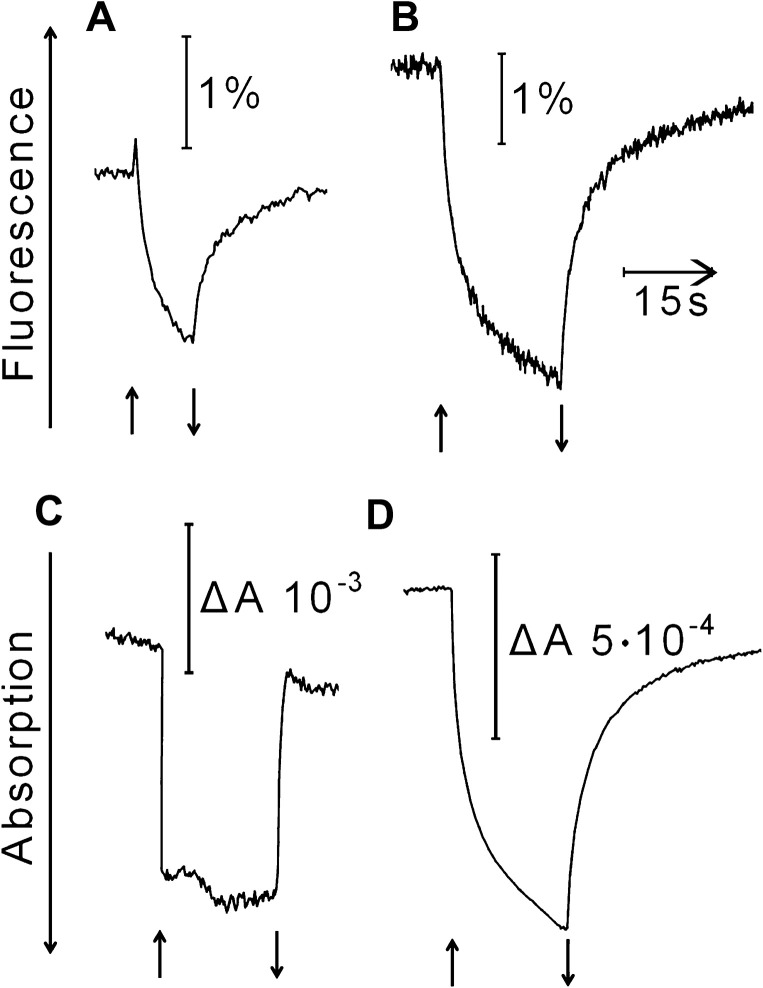
Photochemical activities of desiccated thalli
In Fig. 7, the kinetics of light-induced changes of the fluorescence yield is compared with those of light-induced absorption changes at 802 and 950 nm. In Fig. 7A, the thalli used had been dark-adapted in the hydrated state for 36 h before they were dried in the dark. Upon strong illumination with white light, fluorescence increased briefly showing charge separation in PSII RCs and rapid reduction of QA. Subsequently, fluorescence declined slowly during the illumination. It increased again on darkening. The initial brief increase in fluorescence seen in Fig. 7A was always absent when light-adapted desiccated thalli were illuminated (Fig. 7B). A semi-logarithmic plot of fluorescence intensity against recovery time in the dark revealed two decay phases. The slowest phase obeyed first order kinetics with a half time of 6 s (apparent reaction rate constant of 0.12 s−1). The faster recovery phases could not be resolved well with the present experimental set up.
Fig. 7.
Reversible light-induced changes of the yield of chlorophyll fluorescence (A, B) and of absorption at 950 nm (C) and 802 nm (D) of desiccated thalli of Rhytidium rugosum: (A) dark-adapted thalli, slowly desiccated in darkness; (B–D), light-adapted thalli, slowly desiccated under illumination. Fluorescence changes in A and B are shown as percentages with respect to the total fluorescence yield of desiccated thalli. Start and termination of illumination with photosynthetically active photon flux density 10,000 μmol m−2 s−1 is shown by arrows.
This study always detected reversible light-dependent absorption changes in the far-red region when desiccated thalli were strongly illuminated with white light. Strong far-red illumination did not elicit appreciable absorption changes or fluorescence responses. It is therefore concluded that the fluorescence changes shown in Fig. 7A and B originated mainly from PSII and not from PSI (see also data in Heber et al., 2006a). The time course of the absorption change at 802 nm (Fig. 7D) or 832 nm (not shown) resembled that of the change in fluorescence yield (Fig. 7B). Strong illumination increased absorption of far-red measuring light, and darkening decreased it.
Light-induced increases in absorption were also observed at 950 nm (Fig. 7C) and 875 nm (not shown). They may be attributed to the formation of carotenoid radicals. Their kinetics were different from those observed at 802 and 835 nm. The dark recovery was too fast to be resolved.
Discussion
Mosses occupy a broad spectrum of ecological niches. Within the family Rhytidiaceae, Rhytidium rugosum tolerates full exposure to the sun, whereas a closely related species, Rhytidiadelphus squarrosus (Heber et al., 2006a) is restricted to shaded environments. Whereas both species tolerate desiccation, Rhytidiadelphus squarrosus bleaches slowly when left desiccated in the sun. In contrast, desiccated thalli of Rhytidium rugosum were not damaged even by very high photon fluxes. Figs. 3 and 6 show that slow desiccation severely depresses the Fm and Fo levels of PSII fluorescence of Rhytidium rugosum. This is accompanied by the acceleration of fluorescence decays (Fig. 2). Hydrated thalli proved to be sensitive to strong light (Fig. 3). From the data, it is concluded that three different mechanisms of NPQ protect PSII RCs of Rhytidium rugosum. One of them is active in hydrated thalli. It appears to involve zeaxanthin and is activated by a protonation reaction (Fig. 4). Two other mechanisms are induced by desiccation. One of them is similar to a mechanism detected before in desiccated lichens (Fig. 2; Veerman et al., 2007; Komura et al., 2010; Miyake et al., 2011). The other is based on a photoreaction. This effect can be observed not only in hydrated but also in desiccated moss thalli (Fig. 7; Heber et al., 2006a). It is characterized by light-dependent absorption changes around 800 nm and is thought to represent the formation of a chlorophyll radical which acts as a quencher of excitation energy in RCs of PSII.
Effectiveness of photoprotection
Values of NPQ, commonly used to describe the extent of photo-protective energy dissipation, are derived from experimentally observed changes in Fm. They fail to describe sufficiently the competition between different fates of absorbed light energy in photosynthesis. Therefore, Fo/Fo' ratios were calculated in an attempt to understand competition between radiationless energy dissipation and fluorescence when photochemical use of light for photosynthesis becomes negligible as thalli dry out (Figs. 3 and 6). In hydrated moss thalli, Fo describes a fluorescence situation, in which photochemical light use approaches 100% and thermal energy dissipation is negligible. After desiccation, Fo' describes a very different situation. Thermal energy dissipation has been activated and photochemical light use is essentially absent. This situation lowers fluorescence below the original Fo level. High Fo/Fo' ratios suggest energy dissipation to be so fast in desiccated thalli as to compete successfully with open PSII RCs for trapping excitation energy. This protects the RCs from photodamage.
Further protection is not necessarily required, but can be provided, if needed, by the formation of a quencher of excitation energy within the RCs. The light-dependent formation of such a quencher is demonstrated in Fig. 7. In Fig. 3, desiccation resulted in NPQ values of 4 and 4.3 when thalli were dried in darkness. Under these conditions, the corresponding Fo/Fo' ratios were 2.3 and 2.8, respectively. Charge separation in PSII RCs was strongly, but not completely, suppressed as shown by residual fluorescence responses to strong light pulses. When the thalli were dried in the light, NPQ was 5.6 and Fo/Fo' was 3.9. At such ratios, charge separation in PSII RCs was fully suppressed. Apparently, excitation energy bypassed RCs. It was rapidly converted to thermal energy.
In hydrated plants, charge separation in PSII RCs is known to take 3–5 ps (Zinth and Kaiser, 1993; Holzwarth et al., 2006). Although it is not known whether desiccation changes these values, the high Fo/Fo' ratios in Fig. 3 suggest that the bulk of excitons is rapidly deactivated and converted to thermal energy in competition with the trapping of excitons by functional PSII RCs. In fact, fluorescence decays in the picosecond time scale for desiccated moss reveal very fast energy transfer from the major PSII antenna bands to a far-red band (Fig. 2). In lichens, the quencher is an unidentified far-red absorbing molecule (Veerman et al., 2007; Komura et al., 2010; Miyake et al., 2011). In contrast, Slavov et al. (2011) proposed that desiccation had increased spillover of excitation energy from PSII to PSI, and that fluorescence is quenched by P700+.
The activation of energy dissipation in hydrated moss thalli by CO2 in the experiment of Fig. 4 resulted in an NPQ of 1.2. Fo/Fo' could not be measured because the Fs level measured in Fig. 4 was above the Fo level. The NPQ of 1.9 induced by the actinic illumination during the second hydration in the experiment of Fig. 3B can, therefore, be attributed to zeaxanthin-dependent energy dissipation.
A comparison of NPQ values shows that activation of energy dissipation by protonation or by light cannot increase NPQ as much as desiccation does. Thus, some photo-damage to RCs under excess light appears to be unavoidable in hydrated thalli (Fig. 3) and metabolic repair of damage is required (Aro et al., 1993). Desiccation-induced fluorescence quenching provides stronger photo-protection to the moss Rhytidium (Figs. 3 and 6) and to lichens (Heber et al., 2007; Veerman et al., 2007; Heber, 2008; Komura et al., 2010; Slavov et al., 2011) than the protonation-regulated energy dissipation mechanism.
Picosecond fluorescence decay
Desiccation of Rhytidium decreased fluorescence intensity of the main band at 687 nm more strongly than above 700 nm (Fig. 1). More fluorescence was lost by desiccation under light than in darkness. This observation suggested migration of excitons from the PSII major antenna chlorophylls towards the far-red bands where they are trapped in dissipation centres (Heber and Shuvalov, 2005). For lichens, time-resolved fluorescence analysis has led to the discovery of a long-wavelength quencher, which is coupled to the pigment system of PSII (Veerman et al., 2007; Komura et al., 2010; Miyake et al., 2011).
For the moss Rhytidium, acceleration of the decay of fluorescence by desiccation is shown in Fig. 2. Fluorescence decay of hydrated thalli was faster at 670 nm than at other wavelengths. This fluorescence is thought to be mainly emitted by antenna chlorophylls in PSII. The decay was 4.6-fold accelerated by desiccation (Table 1). At 680–690 nm of PSII range, decay was 4.4-fold accelerated by desiccation. At 720 and 750 nm, where fluorescence is thought to come also from PSI that decays faster, desiccation accelerated the decays only about 3-fold, which is less compared to the acceleration in the PSII range. It is, therefore, concluded that fluorescence of PSII was specifically accelerated by desiccation (Fig. 2A).
In a previous lichen study, it was proposed that a newly activated energy-accepting molecule, which emits fluorescence around 740 nm, accepts excitation energy from PSII bands of shorter wavelengths (Veerman et al., 2007; Komura et al., 2010). The acceleration of the 720 and 750 nm fluorescence decay by desiccation in Fig. 2 suggests that the quencher activated in Rhytidium rugosum is also connected to the PSII antenna. It, therefore, appears that the mechanism of fluorescence quenching in the near far-red detected in the desiccated moss in this study is very similar to that in desiccated lichens. The acceleration of fluorescence decay in the moss is less marked compared to that in lichens.
Co-operation of desiccation-induced energy dissipation centres
Results of Figs. 2, 3, and 7 are interpreted to show cooperation of two different mechanisms of energy dissipation in desiccated Rhytidium. One is characterized by the accelerated decay of 690 nm emission and the light-dependent formation of a quencher presumably in PSII RCs (Fig. 7), the other one by rapid fluorescence decay in the near far-red. Fast loss of 750 nm fluorescence is interpreted to be the result of fast migration of excitons from the PSII antenna to an as yet unknown pigment protein in or near the PSII antenna. There, thermal de-excitation to the ground state takes place.
Special proteins such as the pH-sensitive PsbS protein of higher plants (Li et al., 2004) and the LhcSR3 protein of lower plants (Benente et al., 2010) are known to be essential components of the zeaxanthin-dependent mechanism of photo-protection. Glutaraldehyde, an agent capable of fixing protein structures (Coughlan and Schreiber, 1984), inhibits loss of fluorescence during desiccation of Rhytidium (Fig. 5A and B) and of lichens (Heber, 2008). Inhibition of desiccation-induced energy dissipation by glutaraldehyde demonstrates the involvement of a pigment protein in the mechanism of energy dissipation. The far-red emission observed at room temperature in Figs. 1 and 2 is, therefore, assumed to partially originate from a special pigment protein, which undergoes conformational changes during desiccation, thereby activating dissipation centres.
Energy, not trapped in these centres, can be quenched in PSII RCs that are transformed into dissipation centres (Fig. 7). Fast decay of fluorescence at 690 nm, which is accelerated by desiccation, may originate from the PSII RC core complex (Fig. 2). It is thought to reflect the quenching of excitation energy in PSII RCs. Fig. 7A shows that strong illumination first increased and then decreased fluorescence of dark-adapted desiccated moss. The initial increase, which indicates reduction of QA in PSII RCs (Duysens and Sweers, 1963), was never observed in desiccated moss thalli that had been dried in the light (Fig. 7B). The slow light-dependent loss of fluorescence (Figs. 7A and B) shows formation of a quencher, which appears from its optical properties to be a chlorophyll radical (Fig. 7D; Borg et al., 1970; Fujita et al., 1978). Such radicals can act as quenchers (Faller et al., 2006).
It should be noted that the quencher is unlikely to be P700+ in PSI RC because strong far-red illumination, which oxidizes P700, did not quench fluorescence in desiccated thalli. PSII RCs contain six chlorophylls, two pheophytins, and two β-carotenes in the core D1/D2 subunit moiety. In desiccated plant leaves and in desiccated moss, carotene was oxidized in the light with very low quantum efficiency (Shuvalov and Heber, 2003; Heber et al., 2006b). When oxidized carotene reacts with a neighbouring chlorophyll in a PSII RC, Chl+ is formed. This chlorophyll radical could be the quencher shown in the experiments of Fig. 7B and D (Faller et al., 2006).
Presence of light during desiccation increases phototolerance
In some chlorolichens, the presence of light during desiccation has been shown to increase fluorescence quenching (Heber et al., 2007). In Fig. 1, illumination of thalli of Rhytidium rugosum during desiccation increased the loss of fluorescence. In Fig. 3, NPQ increased from 4 during the desiccation in darkness to 5.6 after desiccation in the light. The Fo/Fo' ratio increased from 2.3 to 3.9. Apparently, the presence of light during the desiccation increased phototolerance considerably.
Levels of zeaxanthin are known to increase under strong illumination when violaxanthin is de-epoxidized in a light-dependent reaction of the xanthophyll cycle (Demmig-Adams, 1990). In a similar de-epoxidation reaction, lutein is synthesized in the light (Matsubara et al., 2007). The photo-protective effect of light during desiccation may suggest a role of zeaxanthin or other carotenoids in the mechanisms of desiccation-induced photoprotection, although inhibitors such as dithiothreitol (Fig. 4) or nigericin (not shown), known to inhibit zeaxanthin-dependent energy dissipation, failed to inhibit desiccation-induced energy dissipation in Rhytidium and in chlorolichens (Heber, 2008).
Molecular mechanisms of energy dissipation
The discussion of molecular mechanisms of energy dissipation is still controversial. In higher plants, energy dissipation is thought to occur in the major light harvesting complex LHC2 of PSII (Pascal et al., 2005) or in the minor antenna proteins such as CP24 or CP29 (de Bianchi et al., 2011). It has been proposed to be the result of aggregation of LHC2 proteins (Horton et al., 1996; Pascal et al., 2005) or of energy transfer from chlorophyll to low-lying electronic states of xanthophylls such as zeaxanthin or lutein (Ruban et al., 2007; Liao et al., 2010). Other proposals envisage the rapid reversible charge transfer from chlorophyll to a xanthophyll (Holt et al., 2005; Avenson et al., 2008) or to chlorophyll (Müller et al., 2010) followed by an energy-dissipating recombination reaction. On the other hand, Slavov et al. (2011) proposed desiccation-induced spillover of excitation energy from PSII to PSI and quenching by P700+ as the main mechanisms of photo-protection in the lichen Parmelia sulcata. A minor role in photo-protection was assigned to desiccation-induced Chl–Chl charge transfer in the antenna of PSII followed by dissipative recombination.
Concluding remarks
The initial aim of the present study was to answer several questions:
(1) Are desiccated thalli of the moss Rhytidium rugosum protected by a mechanism of thermal energy dissipation similar to that previously shown to prevent photo-oxidative damage to desiccated lichens? The answer is yes (Fig. 2).
(2) What is the role of a previously observed radical in the photoprotection of desiccated Rhytidium rugosum and is it stable? The answer is that the radical is a quencher of excitation energy. It is tentatively identified as a chlorophyll radical in PSII RCs, which is produced in a reversible photoreaction even in desiccated moss thalli (Fig. 7).
(3) Is the recombination of unstable radicals such as P680+ with pheo–non-toxic or potentially toxic by giving rise to the activation of oxygen? The answer is that recombination reactions of radical pairs, when they occur, are non-toxic in desiccated thalli of Rhytidium rugosum as shown by full photo-protection of thalli after exposure to extremely strong illumination (Fig. 6). Similar observations reported for desiccated cyanobacteria are explained by a pheophytin-independent recombination pathway (Ohad et al., 2011). Oxygen activation is not observed.
(4) Does the effectiveness of photo-protection of hydrated moss differ from that of desiccated moss? The answer is yes. Hydrated thalli are far more sensitive to damage by strong light than desiccated thalli (Fig. 3).
Acknowledgments
Professor R. Hedrich, Julius-von-Sachs-Institute, University of Würzburg, provided laboratory space and facilities to UH. HY, YF, and SI thank Dr. Masahiro Ishiura, Centre for Gene Research, Nagoya University, and Dr. Tsutomu Kouyama for their kind support of this research.
References
- Aro EM, Virgin I, Andersson B. Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochimica et Biophysica Acta. 1993;1143:113–134. doi: 10.1016/0005-2728(93)90134-2. [DOI] [PubMed] [Google Scholar]
- Asada K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiology. 2006;141:391–396. doi: 10.1104/pp.106.082040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avenson TJ, Ahn TK, Zigmantas D, Niyogi KK, Li Z, Ballottari M, Bassi R, Fleming GR. Zeaxanthin radical cation formation in minor light-harvesting complexes of higher plant antenna. Journal of Biological Chemistry. 2008;283:3550–3558. doi: 10.1074/jbc.M705645200. [DOI] [PubMed] [Google Scholar]
- Bianchi de S, Betterle N, Kouril R, Cazzaniga R, Boekema E, Miloslavina Y, Holzwarth A, Dall ´ Ostro L, Bassi R. Reverse genetics of monomeric Lhcb proteins in Arabidopsis reveals a crucial role for Lhcb4 in photoprotection and defines a multiple quenching model for non-photochemical quenching. 2011 International Workshop “Mechanisms of Non-photochemical Quenching”, Passau, Germany, p. 20. [Google Scholar]
- Bonente G, Ballottari M, Truong TB, Morosinotto T, Ahn TK, Fleming GR, Niyogi KK, Bassi R. Analysis of LhcSR3, a protein essential for feedback de-excitation in the green alga Chlamydomonas reinhardtii . PLOS Biology. 2010;9:e1000577. doi: 10.1371/journal.pbio.1000577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkman O, Demmig B. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta. 1987;170:489–504. doi: 10.1007/BF00402983. [DOI] [PubMed] [Google Scholar]
- Björkman O, Demmig-Adams B. Regulation of photosynthetic light energy capture, conversion and dissipation in leaves of higher plants. In: E-D Schulze, MM Caldwell., editors. Ecophysiology of Photosynthesis. Heidelberg, Germany: Springer Verlag; 1994. pp. 17–70. [Google Scholar]
- Borg DC, Fajer J, Felton RH, Dolphin D. The π-cation radical of chlorophylla. Proceedings of the National Academy of Sciences, USA. 1970;67:813–820. doi: 10.1073/pnas.67.2.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhov NG, Kopecky J, Pfündel EE, Klughammer C, Heber U. A few molecules of zeaxanthin per reaction centre of photosystem II permits effective thermal dissipation of light energy in a poikilohydric moss. Planta. 2001;212:739–748. doi: 10.1007/s004250000485. [DOI] [PubMed] [Google Scholar]
- Coughlan SJ, Schreiber U. The differential effects of short-time glutaraldehyde treatments on light-induced thylakoid membrane conformational changes, proton pumping and electron transport properties. Biochimica et Biophysica Acta. 1984;767:606–617. [Google Scholar]
- Demmig-Adams B. Carotenoids and photoprotection of plants: a role for the xanthophyll zeaxanthin. Biochimica et Biophysica Acta. 1990;1020:1–24. [Google Scholar]
- Duysens LNM, Sweers HE. Mechanism of two photochemical reactions in algae as studied by means of fluorescence. In: J Ashida., editor. Studies on Microalgae and Photosynthetic Bacteria. Tokyo: Tokyo University Press; 1963. pp. 353–372. [Google Scholar]
- Faller P, Fufezan C, Rutherford AW. Side path electron donors: cytochrome b559, chlorophyll Z and β-carotene. In: T Wydrzynski, K Satoh., editors. Photosystem II: the water/plastoquinone oxidoreductase in photosynthesis. Dordrecht: Kluwer; 2006. pp. 347–365. [Google Scholar]
- Fujita I, Davis MS, Fajer J. Anion radicals of pheophytin and chlorophyll a: their role in the primary charge separations of plant photosynthesis. Journal of the American Chemical Society. 1978;100:6280–6282. [Google Scholar]
- Genty B, Briantais J-M, Baker NR. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta. 1989;990:87–92. [Google Scholar]
- Heber U. Photoprotection of green plants: a mechanism of ultra-fast thermal energy dissipation in desiccated lichens. Planta. 2008;228:641–650. doi: 10.1007/s00425-008-0766-5. [DOI] [PubMed] [Google Scholar]
- Heber U, Azarkovich M, Shuvalov V. Activation of mechanisms of photoprotection by desiccation and by light: poikilohydric photoautotrophs. Journal of Experimental Botany. 2007;58:2745–2759. doi: 10.1093/jxb/erm139. [DOI] [PubMed] [Google Scholar]
- Heber U, Bilger W, Shuvalov VA. Thermal energy dissipation in reaction centres and in the antenna of photosystem II protects desiccated poikilohydric mosses against photooxidation. Journal of Experimental Botany. 2006a;57:2993–3006. doi: 10.1093/jxb/erl058. [DOI] [PubMed] [Google Scholar]
- Heber U, Lange OL, Shuvalov VA. Conservation and dissipation of light energy as complementary processes: homoiohydric and poikilohydric autotrophs. Journal of Experimental Botany. 2006b;57:1211–1223. doi: 10.1093/jxb/erj104. [DOI] [PubMed] [Google Scholar]
- Heber U, Shuvalov VA. Photochemical reactions of chlorophyll in dehydrated photosystem II: two chlorophyll forms (680 and 700 nm) Photosynthesis Research. 2005;84:85–91. doi: 10.1007/s11120-005-0413-y. [DOI] [PubMed] [Google Scholar]
- Holt NE, Zigmantas D, Valkunas L, Li X-P, Niyogi KK, Fleming GR. Carotenoid cation formation and the regulation of photosynthetic light harvesting. Science. 2005;307:433–436. doi: 10.1126/science.1105833. [DOI] [PubMed] [Google Scholar]
- Holzwarth AR, Müller MG, Reus M, Nowaczyk M, Sander J, Rögner M. Kinetics and mechanism of electron transfer in intact photosystem II and in the isolated reaction center: pheophytin is the primary electron acceptor. Proceedings of the National Academy of Sciences, USA. 2006;103:6895–6900. doi: 10.1073/pnas.0505371103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton P, Ruban AV, Walters RG. Regulation of light harvesting in green plants. Annual Review of Plant Physiology and Plant Molecular Biology. 1996;47:655–684. doi: 10.1146/annurev.arplant.47.1.655. [DOI] [PubMed] [Google Scholar]
- Komura M, Itoh S. Fluorescence measurement by a streak camera in a single-photon-counting mode. Photosynthesis Research. 2009;101:119–133. doi: 10.1007/s11120-009-9463-x. [DOI] [PubMed] [Google Scholar]
- Komura M, Yamagishi A, Shibata Y, Iwasaki I, Itoh S. Mechanism of strong quenching of photosystem II chlorophyll fluorescence under drought stress in a lichen, Physciella melanchla, studied by subpicosecond fluorescence spectroscopy. Biochimica et Biophysica Acta. 2010;1797:331–338. doi: 10.1016/j.bbabio.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Krieger-Liszkay A. Singlet oxygen production in photosynthesis. Journal of Experimental Botany. 2005;56:337–346. doi: 10.1093/jxb/erh237. [DOI] [PubMed] [Google Scholar]
- Krieger-Liszkay A, Fufezan C, Trebst A. Singlet oxygen production in photosystem II and related protection mechanism. Photosynthesis Research. 2008;98:551–564. doi: 10.1007/s11120-008-9349-3. [DOI] [PubMed] [Google Scholar]
- Lakatos M. 2011. Lichens and bryophytes: habitats and species. In: U Lüttge, E Beck, D Bartels, eds, Plant Desiccation Tolerance. Heidelberg: Springer, pp 65–87. [Google Scholar]
- Li X-P, Gilmore AM, Caffari S, Bassi R, Golan T, Kramer D, Niyogi KK. Regulation of photosynthetic light harvesting involves intrathylakoid lumen pH sensing by the PsbS protein. Journal of Biological Chemistry. 2004;279:22866–22874. doi: 10.1074/jbc.M402461200. [DOI] [PubMed] [Google Scholar]
- Liao PN, Holleboom CP, Wilk L, Kühlbrandt W, Walla PJ. Correlation of Car S1→Chl with Chl→Car S1 energy transfer supports the excitonic model in quenched light harvesting complex II. Journal of Physical Chemistry B. 2010;114:15650–15655. doi: 10.1021/jp1034163. [DOI] [PubMed] [Google Scholar]
- Ma Y-Z, Holt NE, Li X-P, Niyogi KK, Fleming GR. Evidence for direct carotenoid involvement in the regulation of photosynthetic light harvesting. Proceedings of the National Academy of Sciences, USA. 2003;100:4377–4382. doi: 10.1073/pnas.0736959100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara S, Morosinotto T, Osmond CB, Bassi R. Short and long-term operation of the lutein-epoxide cycle in light-harvesting antenna complexes. Plant Physiology. 2007;144:926–941. doi: 10.1104/pp.107.099077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake H, Komura M, Itoh S, Kosugi M, Kashino Y, Satoh K, Shibata Y. Multiple dissipation components of excess light energy in dry lichen revealed by ultrafast fluorescence study at 5 K. Photosynthesis Research. 2011;110:39–48. doi: 10.1007/s11120-011-9691-8. [DOI] [PubMed] [Google Scholar]
- Müller MG, Lambrev P, Reus M, Wientjes E, Croce R, Holzwarth AR. Singlet energy dissipation in the photosystem II light-harvesting complex does not involve energy transfer to carotenoids. Chemical Physics and Physical Chemistry. 2010;11:1289–1296. doi: 10.1002/cphc.200900852. [DOI] [PubMed] [Google Scholar]
- Niyogi KK. Photoprotection revisited: genetic and molecular approaches. Annual Review of Plant Physiology and Plant Molecular Biology. 1999;50:333–359. doi: 10.1146/annurev.arplant.50.1.333. [DOI] [PubMed] [Google Scholar]
- Ohad I, Berg A, Berkowicz SM, Kaplan A, Keren N. Photoinactivation of photosystem II: is there more than one way to skin a cat? Physiologia Plantarum. 2011;142:79–86. doi: 10.1111/j.1399-3054.2011.01466.x. [DOI] [PubMed] [Google Scholar]
- Pascal AA, Liu Z, Broess K, van Oort B, van Amerongen H, Wang C, Horton P, Robert B, Chang W, Ruban A. Molecular basis of photoprotection and control of photosynthetic light-harvesting. Nature. 2005;436:134–137. doi: 10.1038/nature03795. [DOI] [PubMed] [Google Scholar]
- Ruban AV, Berera R, Illioaia C, van Stokkum IHM, Kennis JTM, Pascal AA, van Amerongen H, Robert B, Horton P, van Grondelle R. Identification of a mechanism of photoprotective energy dissipation in higher plants. Nature. 2007;450:575–578. doi: 10.1038/nature06262. [DOI] [PubMed] [Google Scholar]
- Schreiber U, Schliwa U, Bilger W. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynthesis Research. 1986;10:51–62. doi: 10.1007/BF00024185. [DOI] [PubMed] [Google Scholar]
- Shuvalov VA, Heber U. Photochemical reactions in dehydrated photosynthetic organisms, leaves, chloroplasts and photosystem II particles: reversible reduction of pheophytin and chlorophyll and oxidation of β-carotene. Chemical Physics. 2003;294:227–237. [Google Scholar]
- Slavov C, Reus M, Holzwarth AR. Two different mechanisms cooperate in the desiccation-induced excited state quenching in Parmelia lichen. 2011 doi: 10.1021/jp402881f. International Workshop ‘Mechanisms of non-photochemical quenching’, Passau, Germany, p 46. [DOI] [PubMed] [Google Scholar]
- Takizawa K, Cruz JA, Kanazawa A, Kramer DM. The thylakoid proton motive force in vivo. Quantitative, non-invasive probes, energetics, and regulatory consequences of light-induced pmf . Biochimica et Biophysica Acta. 2007;1767:1233–1244. doi: 10.1016/j.bbabio.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Veerman J, Vasil´ev S, Paton GD, Ramanauskas J, Bruce D. Photoprotection in the lichen Parmelia sulcata: the origins of desiccation-induced fluorescence quenching. Plant Physiology. 2007;145:997–1005. doi: 10.1104/pp.107.106872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto HY, Kamite L. The effects of dithiothreitol on violaxanthin de-epoxidation and absorbance changes in the 500 nm region. Biochimica et Biophysica Acta. 1972;267:538–543. doi: 10.1016/0005-2728(72)90182-x. [DOI] [PubMed] [Google Scholar]
- Zinth W, Kaiser W. Time-resolved spectroscopy of the primary electron transfer in reaction centers of Rhodopacter sphaeroides and Rhodopseudomonas viridis . In: J Deisenhofer, JR Norris., editors. The Photosynthetic Reaction Center. San Diego: Academic Press; 1993. pp. 71–88. [Google Scholar]