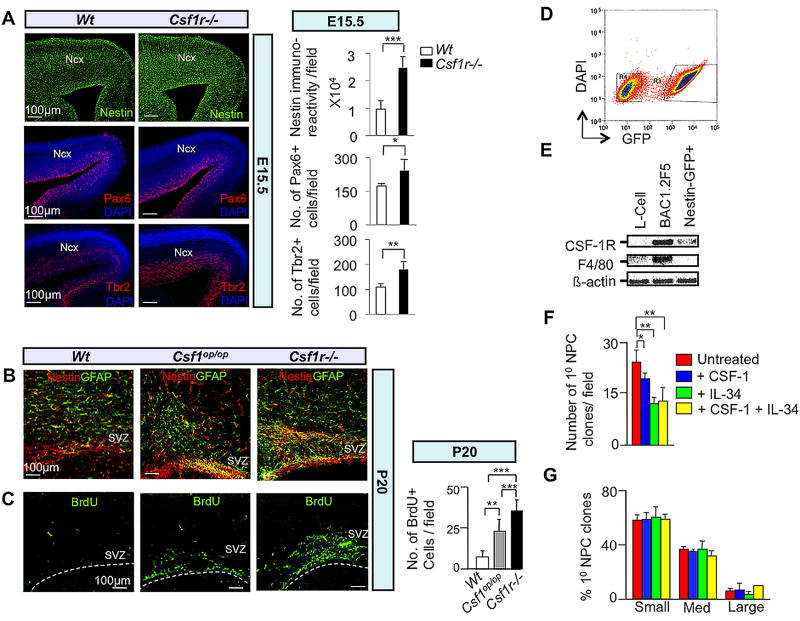
Fig. 3.
The CSF-1R directly mediates suppression of dorsal forebrain progenitor proliferation/self-renewal. (A–C) In vivo studies: Immunofluorescence microscopy of coronal sections of E15.5 (A) and P20 (B,C) Wt and mutant neocortex. (A) Nestin, Pax6 and Tbr2, (B) Nestin-GFAP double and (C) BrdU immunostaining. Quantification= cells/field. Mean ± SD of four representative fields per genotype. n=3: *, P<0.05; **, P<0.01 and ***, P< 0.001. Ncx, neocortex; SVZ, subventricular zone. Dotted lines in (C) delineate the ventricular lining. (D–G) In vitro studies: (D) FACS purification of cerebral cortical GFP+ progenitors from P2 Nestin-GFP transgenic pups. Cells were cultured in the absence of CSF-1 for 3 days and subjected to FACS. Cells gated in region R3 were used to set up neurosphere cultures. (E) Total RNA isolated from the GFP+ fraction was subjected to RT-PCR for assessment of Csf1r and F4/80 (Hume et al., 1983) mRNAs. RNA from L-cells and BAC1.2F5 macrophages, respectively, represent negative and positive controls for F4/80 and Csf1r mRNA expression. (F) FACS-purified GFP+ cells incubated in the presence of EGF and combinations of CSF-1 and IL-34 for 7 DIV. Reduced numbers of primary progenitor clones following incubation with CSF-1 and/or IL-34 for 7 DIV. (G) Failure of CSF-1 or IL-34 to affect the generation of small, medium or large-sized primary clones after 7 DIV. Clonal sizes: Small, 0.5–2.0 mm2; medium, 2.0–6.0 mm2; large, ≥ 6.0 mm2. Mean ± SEM of 16 different representative fields from three independent experiments between untreated and CSF-1, IL-34 and CSF-1+IL-34 treated conditions. *P<0.05, **P<0.01 and ***P<0.001.