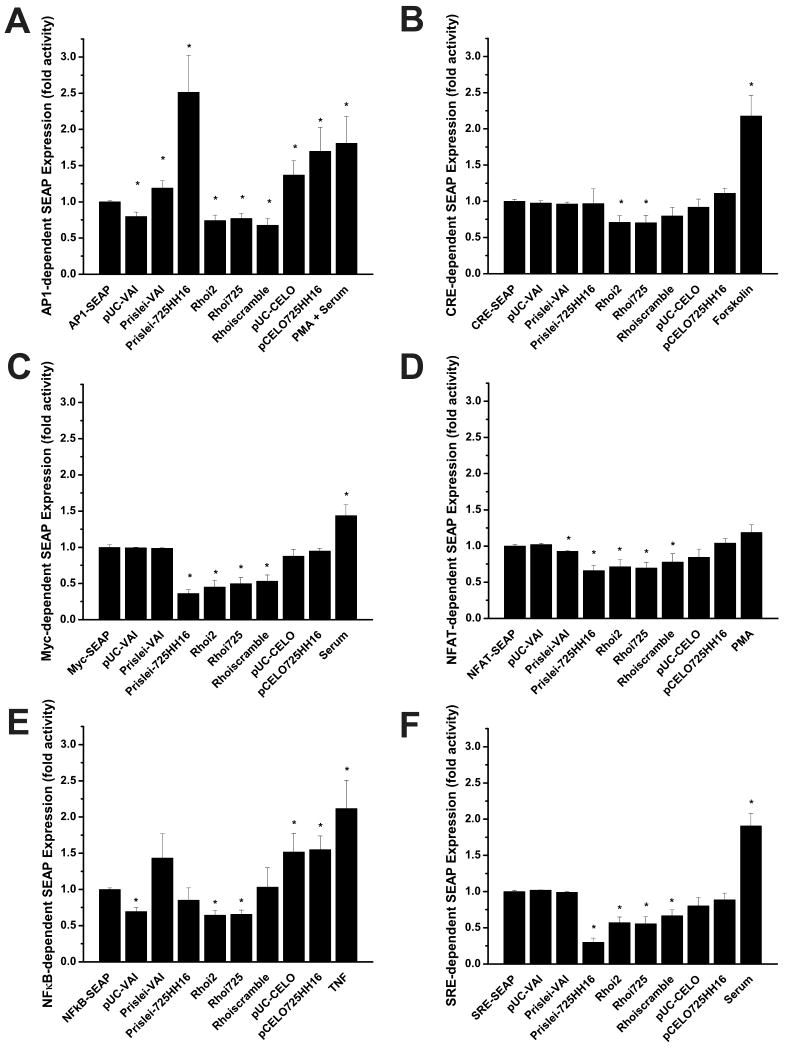
Fig. 4. Transcriptional Activation Assays.
VAI-hhRz chimeras and shRNA targeting accessible regions in human rod opsin mRNA (RHO mRNA) were co-transfected into HEK293S-MSR1 cells with plasmids containing a SEAP reporter gene downstream of different transcription factor binding sequences. Transfection media was removed and replaced at 24 hrs post-transfection. Known activators of each response element were also added as positive controls (Forskolin 20.5 μg/μl; Serum 20% (v/v); TNFα 20 ng/ml; PMA 100 nM). SEAP protein was assayed on aliquots of extracellular growth media at 72 hrs post-transfection. Results are expressed as fold activity over the control and are an average of at least three independent experiments. (A) AP1 testing (ANOVA: F= 5.706, p= 1.05E-6; statistically significant upregulated internal t-test: Prislei-VAI t= 2.052 p= 0.049; Prislei-725HH16 t= 3.249, p= 0.003; pUC-CELO t= 2.653 p= 0.014; pCELO725HH16 t= 3.02, p= 0.006; PMA + Serum t = 2.015, p = 0.051; statistically significant downregulated internal t-test: pUC-VAI t= -3.752 p= 8.131E-4; Rhoi2 t= -3.685 p= 8.695E-4; Rhoi725 t= -3.057 p= 0.004; Rhoiscramble t= -3.828 p= 5.873E-4), (B) CRE testing (ANOVA: F= 9.229, p= 6.775E-12; statistically significant upregulated internal t-test: Forskolin t= 4.12, p= 3.038E-4; statistically significant downregulated internal t-test: Rhoi2 t= -3.105 p= 0.004; Rhoi725 t= -2.853 p= 0.008), (C) Myc testing (ANOVA: F= 14.411, p= 2.909E-14; statistically significant upregulated internal t-test: serum t= 2.533, p= 0.018; statistically significant downregulated internal t-test: Prislei725HH16 t= -9.549 p= 8.07E-10; Rhoi2 t= -5.462 p= 1.735E-5; Rhoi725 t= -5.306 p= 2.518E-5; Rhoiscramble t= -5.052 p= 4.647E-5), (D) NFAT testing (ANOVA: F= 6.357 p= 2.67E-8; statistically significant downregulated internal t-test: Prislei-VAI t= -2.368 p= 0.027; Prislei-725HH16 t= -3.953 p= 4.165E-4; Rhoi2 t= -3.804 p= 7.087E-4; Rhoi725 t= -2.897 p= 0.007; Rhoiscramble t= -2.119 p= 0.044), the PMA positive control had increased SEAP expression but this was not statistically significant (t= 1.761, p = 0.089), (E) NFκB testing (ANOVA: F= 4.909, p= 1.08E-5; statistically significant upregulated internal t-test: pUC-CELO t= 2.886, p= 0.008; pCELO725HH16 t= 4.129, p= 3.56E-4; TNF t= 2.854, p= 0.007; statistically significant downregulated internal t-test: pUC-VAI t= -5.971 p= 1.979E-6; Rhoi2 t= -5.384 p= 7.142E-6; Rhoi725 -5.537 p= 3.445E-6), (F) SRE testing (ANOVA: F= 20.912 p= 0; statistically significant upregulated internal t-test: serum t= 4.606, p=1.036E-4; statistically significant downregulated internal t-test: Prislei-725HH16 t= -9.883 p= 4.063E-10; Rhoi2 t= -5.425 p= 1.893E-5; Rhoi725 t= -4.460 p= 1.96E-4; Rhoiscramble t= -3.969 p= 6.510E-4) * denotes a statistically significant increase (upregulation) or decrease (downregulation) in SEAP activity relative to the control (p ≤ 0.05).