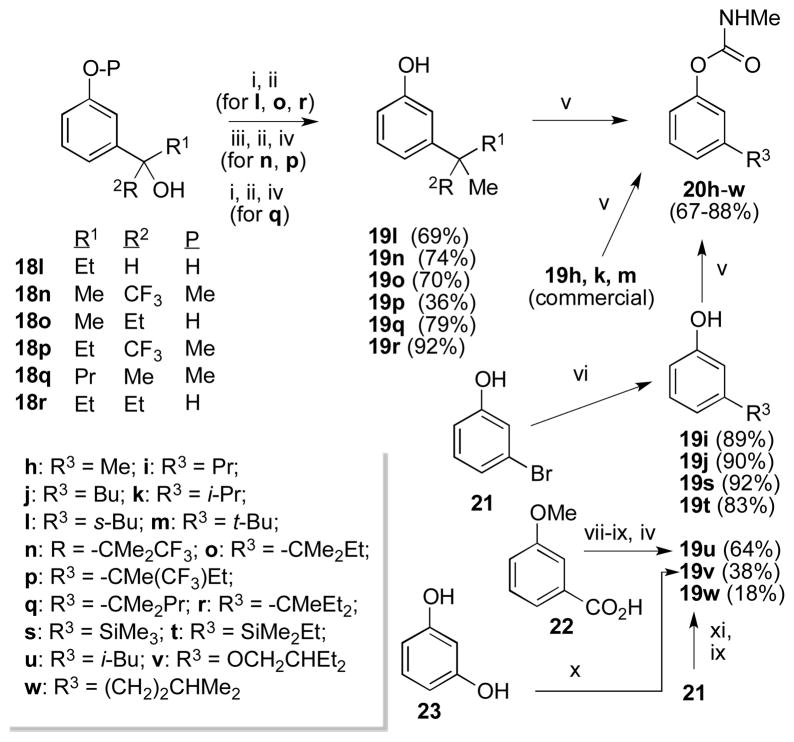
Scheme 2.
Synthesis of 3-substituted aryl methylcarbamates 20h–w.
NHMe i) SOCl2, 0°C. ii) AlMe3, CH2Cl2, 0 °C. iii) NaH, THF, MsCl. iv) BBr3, CH2Cl2.v) KOt-Bu, THF; CH3NHC(O)Cl. vi) 2.1 equiv n-BuLi, THF, −78 to 0 °C; 2 equiv R3-Br or R3-Cl (for 19s, t); aq. HCl. vii) DMAP, EDCI, HN(OMe)Me, CH2Cl2. viii) i-PrMgCl, Et2O, reflux. ix) NH2NH2, EtOH; KOH, ethylene glycol, 160 °C. x) NaOMe, BrCH2CHEt2, MeOH. xi) 2.1 equiv n-BuLi, THF, −78 to 0 °C; Me(MeO)NC(O)CH2CHMe2.