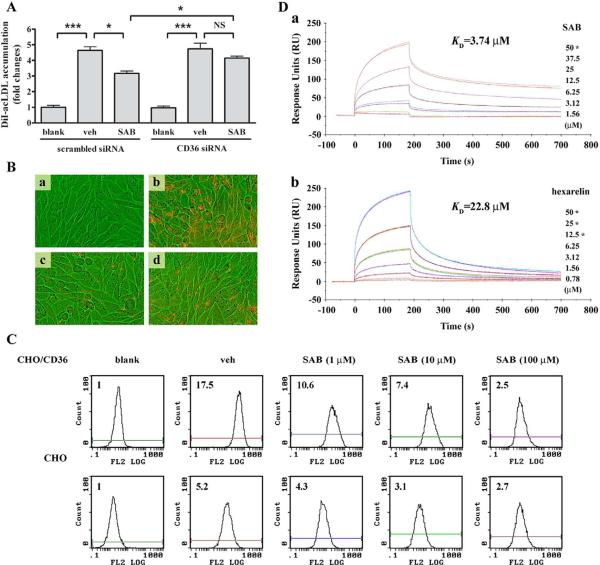
Fig. 2.
SAB-induced inhibition of DiI-acLDL uptake was CD36-dependent. (A) The effect of SAB on DiI-acLDL uptake by PMA-induced THP-1 cells with CD36 siRNA using flow cytometry. The experiment was repeated for 4 times. *, p<0.05, ***, p<0.001 and NS, not significant. (B) The uptake of DiI-acLDL by CHO/CD36 cells. CHO/CD36 cells incubated with DiI-acLDL in the presence of SAB, vehicle or 80 μg/mL acLDL were visualized and photographed using IN Cell Analyzer 1000 with a 20×objective. a, blank cells; b, 2 μg/mL DiI-acLDL treated cells; c, 2 μg/mL DiI-acLDL+100 μM SAB treated cells; d, 2 μg/mL DiI-acLDL+80 μg/mL acLDL treated cells. (C) Flow cytometry analysis for uptake of DiI-acLDL by CHO cells and CHO/CD36 cells. Indicated concentrations of SAB were applied on both cell lines. The uptake of DiI-acLDL was detected in 10,000 cells from the whole portion of each group by FACS. Experiment was repeated 3 times and the representative result was presented. The bar graph reflected the quantified abundance of fluorescence. blank, cells only control; veh, 2 μg/ml DiI-acLDL treated cells; SAB, 2 μg/ml DiI-acLDL and 100 μM SAB treated cells. (D) Surface plasmon resonance (SPR) analysis of the binding of SAB (a) or hexarelin (b) to immobilized CD36. Recombinant human CD36 was immobilized on the surface of a CM5 sensor chip, and indicated concentrations of SAB or hexarelin were injected across the surface. Injections were aligned at t=0. The star symbol (*) means that the duplicate concentration was applied to evaluate the parallelism of data.