Abstract
Alcohol consumption and exposure to stressful life events activate similar neural pathways and thus result in several comparable physiological and behavioral effects. Alcoholics in treatment claim that life-stressors are the leading cause of continued drinking or relapse. However, few studies have investigated the interactive effects of stress and alcohol on cognitive behavior. The effects of restraint stress, alcohol, and stress in combination with alcohol were examined on a spatial memory test, the object placement (OP) task. In addition, intake levels were measured to determine if stress altered general consumption of alcohol. Male Sprague-Dawley rats were assigned to one of four conditions: no alcohol/no stress control (CON), stress alone (STR), alcohol alone (ALC), and STR + alcohol (STR+ALC). Following each restraint stress bout, the STR+ALC and the ALC groups were given access to 8% alcohol for 1-hour using the two-bottle choice limited access paradigm. As predicted,the STR+ALC group significantly increased alcohol consumption, while the ALC group had consistent drinking over the 10-day treatment. On the OP task, STR and ALC groups performed at chance levels, whereas the CON and STR+ALC groups significantly discriminated between objects in the new and old locations. These data show that stress increases alcohol intake and the intake of alcohol is associated with reduction of the stress-induced impairment of spatial memory. The data have important implications for the development of alcohol abuse and its treatment.
Keywords: Alcohol, Stress, Object Placement, Spatial Memory, Limited Access
Introduction
Alcohol consumption and abuse are major problems with devastating social and economic consequences. Initial consumption may lead to one of four possibilities: abstinence, non-problematic use, abuse, or dependence. Chronic alcohol consumption often leads to various aversive consequences such as, memory impairments, anxiety disorders, depression, renal and liver failure, and teratogenic effects (Hoffman and Matthews et al., 2001; Santucci et al., 2008; Seeley, 1960; Berner et al., 1986; Pfefferbaum and Sullivan, 2005). Behavioral and physiological responses to alcohol vary and are often dependent on drinking and family history (Schuckit et al., 1988). Schukit has shown that sons of alcoholic fathers have a reduced stress response to an alcohol intoxication challenge, and may predict future abuse potential. Furthermore, alcoholics in treatment claim that stress is the major factor for their continued drinking and relapse (Sinha, 2001). The relationship between life stressors and alcohol consumption is complex and warrants further investigation.
The “self-medication hypothesis” suggests that an individual may initiate or increase substance use as a way of relieving aversive daily experiences (Khantzian, 1997). Under stressful conditions, animal models of alcohol consumption show a variety of drinking patterns. Various researchers have found reinstatement of alcohol seeking behavior after extinction when foot-shock stress is applied (Liu and Weiss, 2003; Le et al., 2005; Matthews et al., 2008). Using a social model of stress, Pohorecky (2008) found that rats classified as subordinate (high-stress), drink more alcohol than dominant rats during a 23-hour probe period. Restraint stress has been sparsely studied in terms of voluntary drinking in rodent models. However, restraint stress has been extensively used to test cognitive function (Luine et al., 1994; Conrad et al., 1996; Bowman et al., 2009). Lynch et al. (1999) found that when rats were given a choice between alcohol and water following 15 minutes of restraint stress, the stress group drank more alcohol than the non-stressed.
Chronic restraint stress has been repeatedly shown to disrupt cognitive behaviors and alter associated neuronal structures (Luine et al., 2007). Following restraint stress for 6hr/day/21days, male rats show impaired learning and memory on tasks such as the radial arm maze, object placement, and Y-maze (Luine et al., 1994; Conrad et al., 1996; Wright et al., 2005; McLaughlin et al., 2007). Such chronic stress levels reduce dendritic arborization in limbic structures important for memory function (Woolley et al., 1990; Magarinos et al., 1997; McEwen et al., 1999; Luine et al., 2006; Conrad, 2008). However, the negative effects of stress appear to depend on environmental, hormonal, and pharmacological factors. Wright and Conrad (2008) found that an enriched environment prevents the stress-induced memory impairments on the Y-maze and Morris Water Maze compared to stressed rats in standard housing. McLaughlin et al. (2010) showed that ovariectomized rats treated with 17β-Estrodial or cholesterol are spared from dendritic retraction in hippocampal areas caused by 3-weeks of restraint stress. Bisagno et al. (2004) found that chronic injection of amphetamine impairs memory on the object recognition task in female rats; however, when the rats were restraint stressed and given amphetamine the impairments were reversed.
The purpose of this study was to investigate the effects of chronic restraint stress on alcohol consumption and possible interactive effects of stress and alcohol on spatial memory. In accordance with the self-medication hypothesis and Lynch et al. (1999), we predicted that stressed rats would voluntarily consume more alcohol than non-stressed rats. We also investigated whether chronic stress-dependent impairments in spatial memory, in male rats, would be alleviated by alcohol availability following a stressor as has been reported by other combinations with stress (see above references).
Materials and Methods
Subjects
Male Sprague-Dawley rats (~250 g, N = 60) obtained from Harlan-Sprague Dawley were individually housed and kept on a 12h reverse light cycle with lights off at 10:00AM. Standard rat chow and water was available ad libitum. After arrival, rats were allowed to acclimate to the environment for one week before treatments were conducted. Rats were randomly assigned to one of four groups; No stress-No Alcohol control (CON, n=10), Alcohol alone (ALC, n=10), Stress alone (STR, n=20), and a combination of Stress and Alcohol (STR+ALC, n=20). The stress paradigm began after reliable drinking was established (see below). All procedures were approved by Hunter College's Animal Care and Use Committee.
Procedures
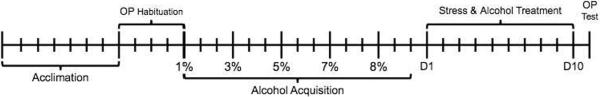
Following arrival and acclimation, rats were trained to prefer alcohol via a two-bottle limited access paradigm (Martinetti et al., 2000). Ethanol was diluted with water to 1%, 3%, 5%, 7%, and 8% v/v. Each concentration was presented in a counterbalanced fashion with water for 1-hour a day for three days. Following the last training day of alcohol, stress treatment began and 8% v/v was available after the stressor for 1-hour for the ALC and STR+ALC groups. The stress and/or alcohol treatments continued for 10 consecutive days. Rats in the CON and STR groups received a 2-bottle choice with both bottles containing only water (See Figure 1 for a timeline of the experimental treatments).
Figure 1.
Time line of procedures. Each tick mark indicates one calendar day.
After the stress and alcohol treatments were complete an object placement (OP) task was conducted. The OP task is a hippocampal dependent spatial memory test designed to measure memory of object location in an open field following a predetermined inter-trial delay (Bowman et al., 2009). Several studies have shown that performance of this task is compromised following hippocampal lesions (Ennaceur and Aggleton, 1994; Ennaceur et al., 1997; Broadbent et al., 2004). The OP task consists of a sample trial (T1), and a retention trial (T2) (Luine et al., 2003). T1 requires two identical objects to be placed at one end of an open field (70 × 70 cm). The rat is allowed to explore the objects for 3-minutes and the amount of time spent exploring each object was recorded with stopwatches. Exploration was defined as whisking, sniffing, and looking at the object from no more than 2 cm away. Following T1, rats are placed back in their home cages left in the experimental room for a 4-hour inter-trial delay. After the delay (T2), one object is relocated to a new position across the open field. The locations of the objects were equidistant from the corners in a diagonal fashion and positioning was counterbalanced. The rat was allowed to explore for 3 minutes and the time spent exploring the objects in the old and new locations was recorded.
Drug Treatment
Rats were administered alcohol via a two-bottle choice limited access paradigm from 12:00 – 1:00PM daily (Martinetti et al., 2000). Ethanol (200 proof, Sigma-Aldrich) was diluted in water and introduced at a concentration of 1% v/v. The percentage of alcohol was gradually increased every three days until rats established reliable drinking of 8% v/v (Figure 1).
Restraint Stress
Restraint stress began after all rats showed stable consumption of ethanol. Rats were restrained, not immobilized, for 1hr/day/10days (11–12PM) in a cylindrical tube constructed of clear Plexiglas measuring 21.5cm long × 6.3cm internal diameter (Harvard Apparatus). One end has a clear, closed end piece containing ventilation holes. The other end has a sliding plastic plug that is secured in place by a screw and adjusted to fit the size of the rat. A slotted opening in the plug allows for free mobility of the tail. Restraint procedures are similar to Lynch et al. (1999) and Bowman et al. (2009). After the restraint stress period, the animals were returned to their home cage and ALC and STR+ALC rats were given a two-bottle choice of 8% ethanol or water for 1-hour (Figure 1).
Statistical Analyses
All statistics were conducted using SPSS (IBM Corp., Somers, NY). A repeated measures ANOVA was used to analyze consumption rates within and between the ALC and STR+ALC groups, and regression analyses was used to determine overall consumption over the course of the stress treatment. A repeated-measures ANOVA was used to analyze exploration times on the object placement task. Significant main effects were further analyzed using paired-t tests (Bonferroni corrected) to determine group performances in ability to discriminate between objects in the New and Old location. A repeated-measures ANOVA was conducted to analyze body weight gain over the treatment period. Significant main effects were further analyzed using a one-way ANOVA and Fisher's Least Significant Difference (LSD) test based on day of treatment.
Results
Alcohol Consumption During Stress
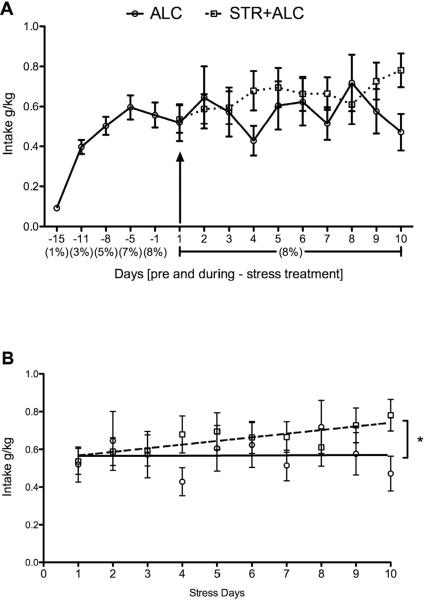
Alcohol consumption was measured daily during the stress period (10 days) in rats treated with or without restraint stress. A repeated-measures two-way ANOVA showed no significant main effects of day [F(9,252)=1.14, p=0.34] and treatment [F(1,28)=0.49, p=0.49], but a significant interaction between day and treatment was found [F(9,252)=2.34, p=0.015] (Figure 2A). The linear regression of drinking behavior over the 10-days of stress (Figure 2B) showed that chronic restraint stress predicts the increased alcohol consumption by the STR+ALC group compared to the ALC group [b=0.495, t(18)=8.54, p<0.0001]. Chronic stress also accounted for the majority of variance associated with the differences between intake values [R2=0.245, F(1,18)=5.85, p=0.026] (Figure 2B).
Figure 2.
(A) Mean alcohol consumption pre- and during-stress treatment. Negative numbers designate days before stress and positive numbers designate days of stress. Numbers in parenthesis show concentration of alcohol (v/v) presented. Arrow denotes first day of stress. (B) Mean alcohol consumption over days of restraint stress. Dashed regression line plotted for STR+ALC group and solid regression line plotted for ALC group. Asterisk (*) indicates a significant difference (R2=0.245, p=0.026) between group regression lines.
Spatial Memory on the Object Placement Task
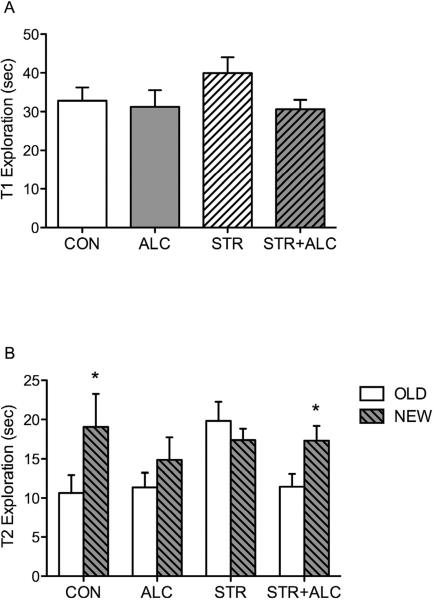
Treatments with alcohol, restraint stress, or the combination of stress and alcohol influenced performance on the object placement task. During the sample trial (T1), an ANOVA showed no significant difference of object exploration time between groups [F(3,56)=1.51, p=0.22] (Figure 3A). However, repeated-measures within subjects analysis of the retention trial (T2) showed a main effect of object [F(1,56)=7.70, p=0.008] and an object by group interaction [F(3,56)=3.42, p=0.02]. A Bonferroni corrected paired-t test was conducted for each group to test for differences in exploration of objects in the old and new locations. Rats in the CON and STR+ALC groups explored the objects in the new location significantly more than objects in the old location [t(9)=3.43 p=0.007; t(19)=2.83, p=0.011, respectively] (Figure 3B). Rats in the ALC and STR groups did not significantly discriminate between objects in the old and new locations [t(9)=1.48, p=0.17; t(19)=0.86, p=.40, respectively] (Figure 3B). Thus, treatment with ALC or STR impairs spatial memory, but the combination of STR+ALC does not.
Figure 3.
(A) Mean exploration time for sample trial (T1) on the object placement task. There was no significant difference between groups (mean = 33 sec.). (B) Following a 4-hour delay T2 shows mean exploration time of objects in the Old and New locations. Asterisk (*) indicates a significant difference (p<0.05) between exploration of the objects in the Old and New locations.
Stress Effects on Body Weight Gain
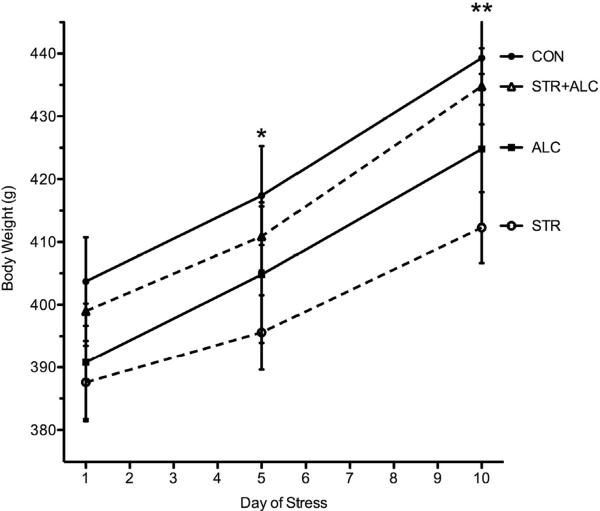
Body weights were analyzed by two-way ANOVA (group × day). There was a significant main effect of day [F(2,112)=320.5, p=0.0001] and significant interaction between day and group [F(6,112)=2.94, p=0.01]. A post-hoc LSD found that by Day 5 the CON group weighed more than the STR group (p=0.04) and by Day 10 both the CON and STR+ALC groups weighed more than the STR group (p=0.015) (Figure 4). On Day 1 of stress, no weight differences between groups were found (M ≈ 395g), and all rats gained weight over the 10-day stress period (M ≈ +35g) (Figure 4). Despite the added calories present in alcohol, rats treated with alcohol did not differ in weight compared to other groups.
Figure 4.
Body weight in grams presented at 5-day intervals. Asterisk (*), significant difference between the CON group and the STR group (p=0.04). Double asterisk (**), significant difference between the CON/STR+ALC groups and the STR group (p=0.015).
Discussion
The current results show that over the period of the stress treatment, rats in the STR+ALC group consume more alcohol than those not stressed. Our findings support others who have found an increase in alcohol self-administration following restraint stress treatment (Lynch et al., 1999; Ploj et al., 2003; Roman et al., 2004) and shows support for the “self-medication” view of increased substance use during times of stress. With respect to spatial memory, alcohol availability after each exposure to stress blocked the impairing effects of treatment with alcohol or stress alone, which is a novel finding and provides rational for self-medicating. The reduction in weight gain, which is a well-known effect of chronic stress, was observed in the STR but not in the STR+ALC group. Despite these findings, it is not entirely clear whether restraint stress is affecting the actions of alcohol or whether alcohol is affecting the stress response.
Alcohol Consumption
Our analyses show that rats in the STR+ALC group significantly increase their alcohol consumption during stress treatment compared to the no-stress ALC group. To elucidate this finding we have to consider previous research using restraint stress and alcohol consumption. Studies using animal models of voluntary consumption have a high amount of variability between results. Previous research on the effects of restraint stress and alcohol consumption has shown a decrease (Sprague and Maickel, 1994; Chester et al., 2004; Boyce-Rustay et al., 2008), increase (Lynch et al., 1999; Pohorecky 2008; Yaroslavsky and Tejani-Butt 2010) or no change (Roske et al., 1994; Rockman and Glavin, 1984; Sillaber et al., 2002) in alcohol intake following stress treatments. Reasons for the high variability between studies may be due to species, type of stress, duration of stress, and availability to alcohol. Even with the few studies that found increased alcohol consumption, there were considerable differences in methodology. Compared to the present study, Ploj et al. (2003) and Roman et al. (2004) used the same alcohol dose, but added maternal separation to their 4-day restraint stressor. Lynch et al. (1999) had procedural differences, but found an increase in consumption on days where alcohol was given via two-bottle choice. However, when rats were forced to drink alcohol (i.e. only fluid), both the stress and non-stressed rats had equal consumption rates. Our data indicate that restraint stress alone increased alcohol consumption. This behavior is often seen in stressed humans and may be associated with the development of dependence over time (Sinha, 2001).
Given the difference in consumption that was found in our experiment, it is pertinent to test whether blood alcohol content (BAC) was altered as well. However, due to a loss of blood samples (defective freezer), we could not directly measure BAC in our study. Future studies will measure BAC to determine if rats undergoing stress altered BAC. This information will help clarify and interpret the current results (the importance of BAC is further discussed below). Additionally, measuring stress hormone levels will provide more information on alcohol's effects on the stress response. Galea et al. (1997) has noted that restraint stress causes a significant increase in plasma corticosterone (CORT) after 30 minutes from Day-1 to Day-21 of restraint. Our study used restraint for 1-hour per day, so it is reasonable to assume that rats in the stress treated groups had higher CORT levels.
Spatial Memory
Numerous experiments have shown that following chronic stress, male rats exhibit impaired memory in tasks such as the radial arm maze, Y-maze, object placement, and water maze (Luine et al., 1994; Conrad et al., 1996; Beck and Luine, 2002; Kitraki et al., 2004). Chronic alcohol consumption has also been shown to impair memory in tasks like the radial arm maze, object recognition, and water maze (Steigerwald and Miller, 1997; Ryabinin et al., 2002; Matthews and Morrow, 2000). Thus, our findings are consistent with previous work showing that chronic stress or alcohol impairs spatial memory on the object placement task. However, when the two treatments are combined (STR+ALC), no deficits in spatial memory are present. Thus, the results do not support additive effects of stress and alcohol on memory. To our knowledge no study has examined spatial memory using low-stress tasks when alcohol is accessible for voluntary consumption following the stressor. In addition, other studies examining stress/alcohol interactions have utilized different spatial memory tasks than employed here. Sircar and Sircar (2005) utilized the Morris Water Maze and Maier and Pohorecky (1986) utilized the radial arm maze. These tasks expose subjects to stressful circumstances (forced swim and food deprivation), which may have confounded testing the interaction between stress and alcohol (Eglemann et al., 2006). Object placement does not use positive or negative reinforcements, and rats undergo habituation trials so that stress is minimal when memory is assessed. We know that chronic exposure to corticosterone impairs spatial memory in male rats (Luine et al., 1993; McLay et al., 1998; Wright et al., 2006). Therefore, it is important to measure corticosterone levels following stress and alcohol treatments to determine the affect on the stress response. There may be a value in interpreting some of these findings such as the possibility that alcohol consumption reduces the negative effects of chronic stress on spatial memory.
Body Weight Gain
As a physiological measure of stress effects, rats were weighed at the beginning, middle and end of the stress treatment. During chronic stress, rats show a reduction of their weight gain, which rebounds once the stressor is terminated (Baran et al. 2005; Conrad et al, 2007; McLaughlin et al., 2007). Our data are consistent with these observations; the STR group gained weight at a lower rate than those in the CON or STR+ALC groups. Hence, when alcohol is available after a stressor, the effect of stress on weight gain is normalized to that of rats in the CON group. Here, we should consider the possibility that alcohol, which has calories, is responsible for the added weight. However, this possibility appears unlikely because rats in the ALC group did not differ from CON, STR, or STR+ALC groups in body weight. Thus, it appears that the intake of alcohol reduced stress-induced reduction in weight gain.
Conclusions
We have found that combination of chronic restraint stress and voluntary alcohol consumption in male rats is associated with increases in alcohol intake, reduced impairment of spatial memory produced by each treatment alone, and inhibition of stress-induced weight loss. These findings are novel and may provide some insight into the complex mechanisms that motivate alcohol abuse and lead to improved treatment for alcohol dependence. However, the mechanisms responsible for the maintenance of spatial memory function at control levels in STR+ALC treated rats are unknown. One explanation is that stress enhances alcohol metabolism, which leads to a decrease in alcohol's effects (Ryabinin et al., 1995; Ryabinin et al., 1999). In our study it is unknown whether alcohol metabolism was affected by stress because alcohol levels were not measured in the subjects; however, preliminary data from ongoing experiments has shown that blood alcohol levels are not altered following 7 days of chronic restraint stress (Gomez et al., 2011). Another mechanism, which may be responsible for the inter-active effects of the treatments on spatial memory, is that alcohol may be dampening the stress response (Spencer and McEwen, 1990; Lee and Rivier, 1999; Richardson et al., 2008). We have found some support for this mechanism in that STR+ALC treated rats showed a significant reduction of corticosterone levels from day-1 to day-7 of restraint stress (Gomez et al., 2011) whereas rats in the other groups (CON, ALC, and STR) showed no change in corticosterone levels.
These data show that restrained stressed rats seek alcohol solutions and that alcohol intake can reduce the consequences of the stress response on weight gain and memory function. Therefore, it appears likely that alcohol consumption may reduce the aversive effects of stress such as anxiety and stress hormone levels. Our current research focuses on these measures to understand how the combination of two impairing manipulations can result in a return to normal memory function. The physiological and neural mechanisms underlying these changes are currently unknown and are the focus of our ongoing research.
Acknowledgements
This study was funded in part by NIH MBRS-RISE: GM060665-12 and grant number RR03037 from the National Center for Research Resources (NCRR). Special thanks to Dr. Martin Chodorow for assistance with statistical analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baran SE, Campbell AM, Kleen JK, Foltz CH, Wright RL, Diamond DM, Conrad CD. Combination of high fat diet and chronic stress retracts hippocampal dendrites. Neuroreport. 2005;16:39–43. doi: 10.1097/00001756-200501190-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck KD, Luine VN. Sex differences in behavioral and neurochemical profiles after chronic stress: role of housing conditions. Physiol. Behav. 2002;75:661–673. doi: 10.1016/s0031-9384(02)00670-4. [DOI] [PubMed] [Google Scholar]
- Berner P, Lesch OM, Walter H. Alcohol and depression. Psychopathology. 1986;19(Suppl 2):177–183. doi: 10.1159/000285152. [DOI] [PubMed] [Google Scholar]
- Bisagno V, Grillo CA, Piroli GG, Giraldo P, McEwen B, Luine VN. Chronic stress alters amphetamine effects on behavior and synaptophysin levels in female rats. Pharmacol. Biochem. Behav. 2004;78:541–550. doi: 10.1016/j.pbb.2004.04.023. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Micik R, Gautreaux C, Fernandez L, Luine VN. Sex-dependent changes in anxiety, memory, and monoamines following one week of stress. Physiol. Behav. 2009;97:21–29. doi: 10.1016/j.physbeh.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Janos AL, Holmes A. Effects of chronic swim stress on EtOH-related behaviors in C57BL/6J, DBA/2J and BALB/cByJ mice. Behav. Brain Res. 2008;186:133–137. doi: 10.1016/j.bbr.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent NJ, Squire LR, Clark RE. Spatial memory, recognition memory, and the hippocampus. Proc. Natl. Acad. Sci. USA. 2004;101:14515–14520. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester JA, Blose AM, Zweifel M, Froehlich JC. Effects of stress on alcohol consumption in rats selectively bred for high or low alcohol drinking. Alcohol. Clin. Exp. Res. 2004;28:385–393. doi: 10.1097/01.alc.0000117830.54371.7a. [DOI] [PubMed] [Google Scholar]
- Conrad CD. Chronic stress-induced hippocampal vulnerability: the glucocorticoid vulnerability hypothesis. Rev. Neurosci. 2008;19:395–411. doi: 10.1515/revneuro.2008.19.6.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, Galea LA, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav. Neurosci. 1996;110:1321–1334. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- Conrad CD, McLaughlin KJ, Harman JS, Foltz C, Wieczorek L, Lightner E, Wright RL. Chronic glucocorticoids increase hippocampal vulnerability to neurotoxicity under conditions that produce CA3 dendritic retraction but fail to impair spatial recognition memory. J. Neurosci. 2007;27:8278–8285. doi: 10.1523/JNEUROSCI.2121-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann M, Ebner K, Landgraf R, Wotjak CT. Effects of Morris water maze testing on the neuroendocrine stress response and intrahypothalamic release of vasopressin and oxytocin in the rat. Horm. Behav. 2006;50:496–501. doi: 10.1016/j.yhbeh.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Aggleton JP. Spontaneous recognition of object configurations in rats: effects of fornix lesions. Exp. Brain. Res. 1994;100:85–92. doi: 10.1007/BF00227281. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Aggleton JP. The effects of neurotoxic lesions of the perirhinal cortex combined to fornix transection on object recognition memory in the rat. Behav. Brain Res. 1997;88:181–193. doi: 10.1016/s0166-4328(97)02297-3. [DOI] [PubMed] [Google Scholar]
- Galea LA, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 1997;81:689–697. doi: 10.1016/s0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- Gomez JL, Lewis M, Frankfurt M, Serrano P, Luine V. Program No. 469.11. 2011 Neuroscience Meeting Planner. Society for Neuroscience; Washington, DC: 2011. Post-stress alcohol treatment alleviates stress-induced impairment of memory in male rats. Online. [Google Scholar]
- Hoffmann SE, Matthews DB. Ethanol-induced impairments in spatial working memory are not due to deficits in learning. Alcohol. Clin. Exp. Res. 2001;25:856–861. [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of substance use disorders: a reconsideration and recent applications. Harv. Rev. Psychiatry. 1997;4:231–244. doi: 10.3109/10673229709030550. [DOI] [PubMed] [Google Scholar]
- Kitraki E, Kremmyda O, Youlatos D, Alexis M, Kittas C. Spatial performance and corticosteroid receptor status in the 21-day restraint stress paradigm. Ann. N. Y. Acad. Sci. 2004;1018:323–327. doi: 10.1196/annals.1296.039. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Funk D, Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology (Berl) 2005;179:366–373. doi: 10.1007/s00213-004-2036-y. [DOI] [PubMed] [Google Scholar]
- Lee S, Kim CK, Rivier C. Nitric oxide stimulates ACTH secretion and the transcription of the genes encoding for NGFI-B, corticotropin-releasing factor, corticotropin-releasing factor receptor type 1, and vasopressin in the hypothalamus of the intact rat. J. Neurosci. 1999;19:7640–7647. doi: 10.1523/JNEUROSCI.19-17-07640.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Weiss F. Stimulus conditioned to foot-shock stress reinstates alcohol-seeking behavior in an animal model of relapse. Psychopharmacology (Berl) 2003;168:184–191. doi: 10.1007/s00213-002-1267-z. [DOI] [PubMed] [Google Scholar]
- Luine V, Attalla S, Mohan G, Costa A, Frankfurt M. Dietary phytoestrogens enhance spatial memory and spine density in the hippocampus and prefrontal cortex of ovariectomized rats. Brain Res. 2006;1126:183–187. doi: 10.1016/j.brainres.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Luine V, Villegas M, Martinez C, McEwen BS. Stress-dependent impairments of spatial memory. Role of 5-HT. Ann. N. Y. Acad. Sci. 1994;746:403–404. doi: 10.1111/j.1749-6632.1994.tb39268.x. [DOI] [PubMed] [Google Scholar]
- Luine VN, Beck KD, Bowman RE, Frankfurt M, Maclusky NJ. Chronic stress and neural function: accounting for sex and age. J. Neuroendocrinol. 2007;19:743–751. doi: 10.1111/j.1365-2826.2007.01594.x. [DOI] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- Luine VN, Spencer RL, McEwen BS. Effects of chronic corticosterone ingestion on spatial memory performance and hippocampal serotonergic function. Brain Res. 1993;616:65–70. doi: 10.1016/0006-8993(93)90193-q. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Kushner MG, Rawleigh JM, Fiszdon J, Carroll ME. The effects of restraint stress on voluntary ethanol consumption in rats. Exp. Clin. Psychopharmacol. 1999;7:318–323. doi: 10.1037//1064-1297.7.4.318. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, Verdugo JM, McEwen BS. Chronic stress alters synaptic terminal structure in hippocampus. Proc. Natl. Acad. Sci. USA. 1997;94:14002–14008. doi: 10.1073/pnas.94.25.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier DM, Pohorecky LA. The effect of ethanol and sex on radial arm maze performance in rats. Pharmacol. Biochem. Behav. 1986;25:703–709. doi: 10.1016/0091-3057(86)90374-6. [DOI] [PubMed] [Google Scholar]
- Martinetti MP, Andrzejewski ME, Hineline PN, Lewis MJ. Ethanol consumption and the matching law: a choice analysis using a limited-access paradigm. Exp. Clin. Psychopharmacol. 2000;8:395–403. doi: 10.1037//1064-1297.8.3.395. [DOI] [PubMed] [Google Scholar]
- Matthews DB, Morrow AL. Effects of acute and chronic ethanol exposure on spatial cognitive processing and hippocampal function in the rat. Hippocampus. 2000;10:122–130. doi: 10.1002/(SICI)1098-1063(2000)10:1<122::AID-HIPO13>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Matthews DB, Morrow AL, O'Buckley T, Flanigan TJ, Berry RB, Cook MN, Mittleman G, Goldowitz D, Tokunaga S, Silvers JM. Acute mild footshock alters ethanol drinking and plasma corticosterone levels in C57BL/6J male mice, but not DBA/2J or A/J male mice. Alcohol. 2008;42:469–476. doi: 10.1016/j.alcohol.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Tanapat P, Weiland NG. Inhibition of dendritic spine induction on hippocampal CA1 pyramidal neurons by a nonsteroidal estrogen antagonist in female rats. Endocrinology. 1999;140:1044–1047. doi: 10.1210/endo.140.3.6570. [DOI] [PubMed] [Google Scholar]
- McLaughlin KJ, Gomez JL, Baran SE, Conrad CD. The effects of chronic stress on hippocampal morphology and function: an evaluation of chronic restraint paradigms. Brain Res. 2007;1161:56–64. doi: 10.1016/j.brainres.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KJ, Wilson JO, Harman J, Wright RL, Wieczorek L, Gomez J, Korol DL, Conrad CD. Chronic 17beta-estradiol or cholesterol prevents stress-induced hippocampal CA3 dendritic retraction in ovariectomized female rats: possible correspondence between CA1 spine properties and spatial acquisition. Hippocampus. 2010;20:768–786. doi: 10.1002/hipo.20678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLay RN, Freeman SM, Zadina JE. Chronic corticosterone impairs memory performance in the Barnes maze. Physiol. Behav. 1998;63:933–937. doi: 10.1016/s0031-9384(97)00529-5. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. Disruption of brain white matter microstructure by excessive intracellular and extracellular fluid in alcoholism: evidence from diffusion tensor imaging. Neuropsychopharmacology. 2005;30:423–432. doi: 10.1038/sj.npp.1300623. [DOI] [PubMed] [Google Scholar]
- Ploj K, Roman E, Nylander I. Long-term effects of maternal separation on ethanol intake and brain opioid and dopamine receptors in male Wistar rats. Neuroscience. 2003;121:787–799. doi: 10.1016/s0306-4522(03)00499-8. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA. Psychosocial stress and chronic ethanol ingestion in male rats: effects on elevated plus maze behavior and ultrasonic vocalizations. Physiol. Behav. 2008;94:432–447. doi: 10.1016/j.physbeh.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Lee SY, O'Dell LE, Koob GF, Rivier CL. Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. Eur. J. Neurosci. 2008;28:1641–1653. doi: 10.1111/j.1460-9568.2008.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockman GE, Glavin GB. Ethanol-stress interaction: differences among ethanol-preferring rats' responses to restraint. Alcohol. 1984;1:293–295. doi: 10.1016/0741-8329(84)90051-x. [DOI] [PubMed] [Google Scholar]
- Roman E, Ploj K, Nylander I. Maternal separation has no effect on voluntary ethanol intake in female Wistar rats. Alcohol. 2004;33:31–39. doi: 10.1016/j.alcohol.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Roske I, Baeger I, Frenzel R, Oehme P. Does a relationship exist between the quality of stress and the motivation to ingest alcohol? Alcohol. 1994;11:113–124. doi: 10.1016/0741-8329(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Melia KR, Cole M, Bloom FE, Wilson MC. Alcohol selectively attenuates stress-induced c-fos expression in rat hippocampus. J. Neurosci. 1995;15:721–730. doi: 10.1523/JNEUROSCI.15-01-00721.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryabinin AE, Miller MN, Durrant S. Effects of acute alcohol administration on object recognition learning in C57BL/6J mice. Pharmacol. Biochem. Behav. 2002;71:307–312. doi: 10.1016/s0091-3057(01)00661-x. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Wang YM, Freeman P, Risinger FO. Selective effects of alcohol drinking on restraint-induced expression of immediate early genes in mouse brain. Alcohol. Clin. Exp. Res. 1999;23:1272–1280. doi: 10.1111/j.1530-0277.1999.tb04288.x. [DOI] [PubMed] [Google Scholar]
- Santucci AC, Cortes C, Bettica A, Cortes F. Chronic ethanol consumption in rats produces residual increases in anxiety 4 months after withdrawal. Behav. Brain Res. 2008;188:24–31. doi: 10.1016/j.bbr.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Risch SC, Gold EO. Alcohol consumption, ACTH level, and family history of alcoholism. Am. J. Psychiatry. 1988;145:1391–1395. doi: 10.1176/ajp.145.11.1391. [DOI] [PubMed] [Google Scholar]
- Seeley JR. Death by liver cirrhosis and the price of beverage alcohol. Can. Med. Assoc. J. 1960;83:1361–1366. [PMC free article] [PubMed] [Google Scholar]
- Sillaber I, Rammes G, Zimmermann S, Mahal B, Zieglgansberger W, Wurst W, Holsboer F, Spanagel R. Enhanced and delayed stress-induced alcohol drinking in mice lacking functional CRH1 receptors. Science. 2002;296:931–933. doi: 10.1126/science.1069836. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sircar R, Sircar D. Adolescent rats exposed to repeated ethanol treatment show lingering behavioral impairments. Alcohol. Clin. Exp. Res. 2005;29:1402–1410. doi: 10.1097/01.alc.0000175012.77756.d9. [DOI] [PubMed] [Google Scholar]
- Spencer RL, McEwen BS. Adaptation of the hypothalamic-pituitary-adrenal axis to chronic ethanol stress. Neuroendocrinology. 1990;52:481–489. doi: 10.1159/000125632. [DOI] [PubMed] [Google Scholar]
- Sprague JE, Maickel RP. Effects of stress and ebiratide (Hoe-427) on free-choice ethanol consumption: comparison of Lewis and Sprague-Dawley rats. Life Sci. 1994;55:873–878. doi: 10.1016/0024-3205(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Steigerwald ES, Miller MW. Performance by adult rats in sensory-mediated radial arm maze tasks is not impaired and may be transiently enhanced by chronic exposure to ethanol. Alcohol. Clin. Exp. Res. 1997;21:1553–1559. [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J. Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RL, Conrad CD. Chronic stress leaves novelty-seeking behavior intact while impairing spatial recognition memory in the Y-maze. Stress. 2005;8:151–154. doi: 10.1080/10253890500156663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RL, Conrad CD. Enriched environment prevents chronic stress-induced spatial learning and memory deficits. Behav. Brain Res. 2008;187:41–47. doi: 10.1016/j.bbr.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RL, Lightner EN, Harman JS, Meijer OC, Conrad CD. Attenuating corticosterone levels on the day of memory assessment prevents chronic stress-induced impairments in spatial memory. Eur. J. Neurosci. 2006;24:595–605. doi: 10.1111/j.1460-9568.2006.04948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaroslavsky I, Tejani-Butt SM. Voluntary alcohol consumption alters stress-induced changes in dopamine-2 receptor binding in Wistar-Kyoto rat brain. Pharmacol. Biochem. Behav. 2010;94:471–476. doi: 10.1016/j.pbb.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]