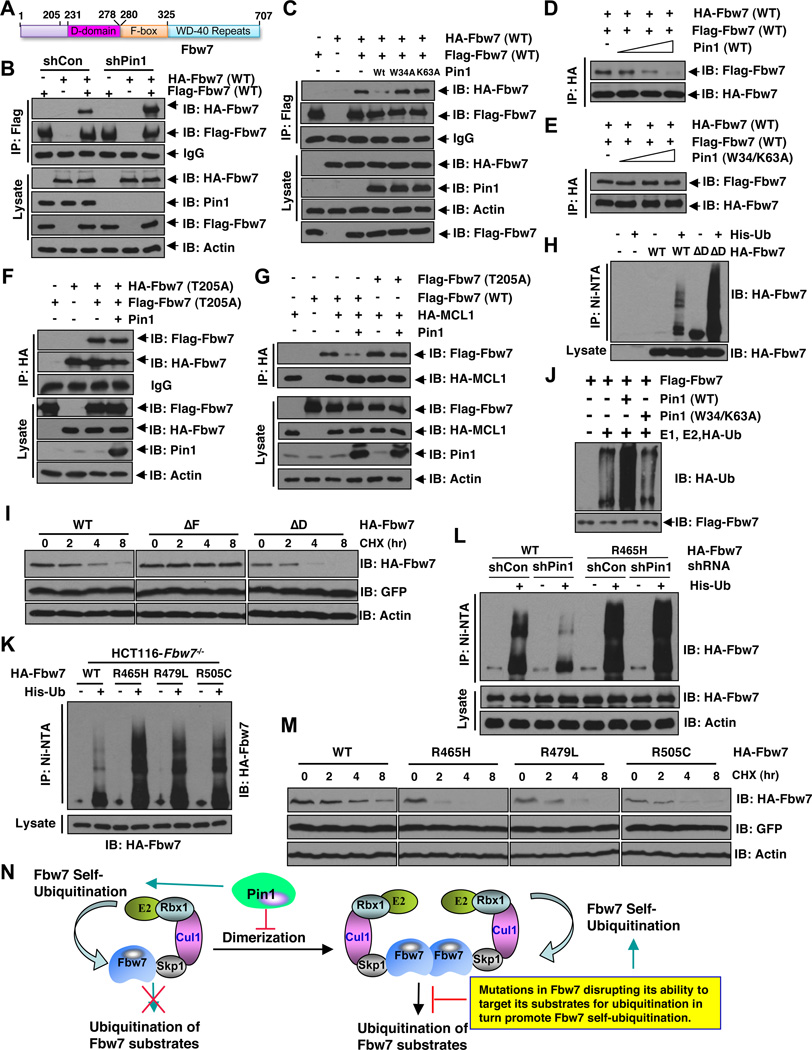
Figure 4. Pin1 Promotes Fbw7 Self-Ubiquitination By Dirupting Fbw7 Dimerization.
A. Schematic representation of the identified D-domain and F-box motif in Fbw7.
B. Stable Pin1 shRNA infected HCT116 Fbw7−/− cells (or shGFP cells as a negative control) were transfected with Flag-Fbw7 and HA-Fbw7 constructs or vector control as indicated, followed by immunoprecipitation with Flag-agarose beads and subjected to immunoblot analysis with the indicated antibodies.
C. Stable Pin1 shRNA infected HCT116 Fbw7−/− cells were transfected with Flag-Fbw7 and HA-Fbw7 constructs in the presence or absence of Pin1 (with empty vector or the indicated Pin1 mutants as negative controls), followed by immunoprecipitation with Flag-agarose beads and subjected to immunoblot analysis with the indicated antibodies.
D–E. HCT116 Fbw7−/− cells were transfected with either a HA-Fbw7 or a Flag-Fbw7 construct. 40 hours post-transfection, anti-Flag immunoprecipitation was performed to affinity-purify Fbw7 protein. The Flag-Fbw7/HA-Fbw7 complex was eluted out with 3xFlag peptide and 0.3 µM of eluted Fbw7 was incubated with increasing doses (0, 0.3, 1 or 3 µM) of recombinant WT-Pin1 (D) or W34/K63A-mutant Pin1 (E) before performing the anti-HA immunoprecipitation. The recovered Fbw7 dimer was monitored by immunoblot analysis.
F. Stable Pin1 shRNA infected HCT116 Fbw7−/− cells were transfected with Flag-Fbw7-T205A and HA-Fbw7-T205A constructs in the presence or absence of Pin1 (with empty vector as a negative control as indicated), followed by immunoprecipitation with HA-agarose beads and subjected to immunoblot analysis with the indicated antibodies.
G. Expression of Pin1 leads to reduced in vivo co-immunoprecipitation between Mcl-1 and ectopically expressed WT-Fbw7, but not T205A-Fbw7. HCT116-Fbw7−/− cells were co-transfected with the indicated Flag-Fbw7, HA-Mcl-1 and Pin1 constructs and then subjected to immunoprecipitation with anti-HA, followed by immunoblotting with the indicated antibodies.
H. Deletion of the D-domain results in increased in vivo Fbw7 ubiquitination. HCT116 cells were transfected with the indicated HA-Fbw7 and/or His-tagged ubiquitin or vector control as indicated, followed by immunoprecipitation with nickel-agarose beads and subjected to immunoblot analysis with anti-HA antibody.
I. Deletion of the F-box motif extended Fbw7 half-life while deletion of the D-domain shortened Fbw7 half-life. HCT116 cells were transfected with wild type, ΔF, or ΔD HA-Fbw7 and Fbw7 stability was then determined using cycloheximide chase.
J. HCT116 Fbw7−/− cells were transfected with a Flag-Fbw7 construct. 40 hours post-transfection, anti-Flag immunoprecipitation was performed to affinity-purify the SCFFbw7 complex followed by elution with 3×Flag peptides. 0.3 µM of affinity purified and eluted SCFFbw7 complex was then incubated with recombinant E1, E2, HA-Ubiquitin protein in the presence of 1 µM of purified recombinant WT-Pin1 or the W34/K63A-mutant Pin1. Fbw7 self-ubiquitination was monitored by immunoblot analysis with the indicated antibodies.
K. T-ALL derived hot-spot Fbw7 mutants displayed increased in vivo Fbw7 ubiquitination. HCT116-Fbw7−/− cells were transfected with the indicated HA-Fbw7 constructs and/or His-tagged ubiquitin or vector control as indicated, followed by immunoprecipitation with nickel-agarose beads and subjected to immunoblot analysis with anti-HA antibody.
L. Depletion of endogenous Pin1 leads to reduced self-ubiquitination of WT-Fbw7, but not R465H-Fbw7. HCT116 Fbw7−/− cells were infected with the indicated shRNA lentiviral vectors and selected in 1 µg/ml puromycin for 72 hours to eliminate the non-infected cells. Afterwards, the generated cells were transfected with the indicated HA-Fbw7 and/or His-tagged ubiquitin or vector control as indicated, followed by immunoprecipitation with nickel-agarose beads and subjected to immunoblot analysis with anti-HA antibody.
M. T-ALL derived hot-spot Fbw7 mutants displayed shortened Fbw7 half-life. HCT116 cells were transfected with the indicated HA-Fbw7 constructs and Fbw7 stability was then determined using cycloheximide chase.
N. A proposed model for Pin1 regulation of Fbw7 self-ubiquitination and stability via inhibiting Fbw7 dimer formation. In addition, we also propose that mutations in Fbw7 that disrupt its ability to ubiquitinate its downstream substrates will phenocopy Pin1 overexpression in promoting Fbw7 self-ubiquitination and subsequent degradation.
(See also Figure S4)