Table 1.
Structures and activities of analogs 11.
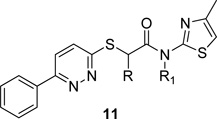 | ||||
|---|---|---|---|---|
| Cmpd | R | R1 | % Inhib. @ 2 µM |
IC50 (nM) |
| 2 | H | Me | 82 | 537 |
| 11a | (±)-Me | Me | 46 | ND |
| 11b | (±)-Et | Me | 27 | ND |
| 11c | (±)-Et | Et | 45 | ND |
| 11d | (±)-Me | Et | 57 | ND |
| 11e | (±)-Me | 87 | 570 | |
| 11f | a(±)-Me | ND | 152 | |
| 11g | a(−)-Me | ND | 1,900 | |
| 11h | (±)-Et | 84 | 756 | |
| 11i | a(+)-Et | ND | 385 | |
| 11j | a(−)-Et | 40 | ND | |
| 11k | H | ND | 61 | |
| 11m | H | ND | 177 | |
| 11n | H | ND | 1057 | |
ND: not determined.
enantiomers separated by chiral SFC and (+) or (−) rotation noted,19 absolute stereochemistry is unknown.
