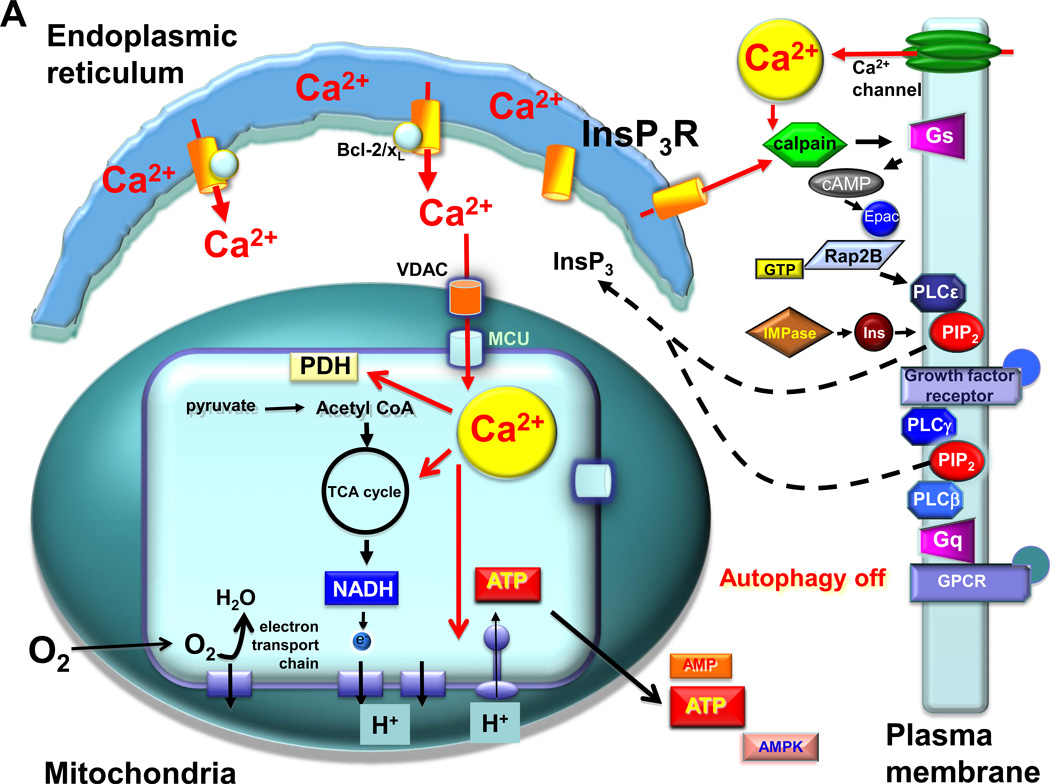
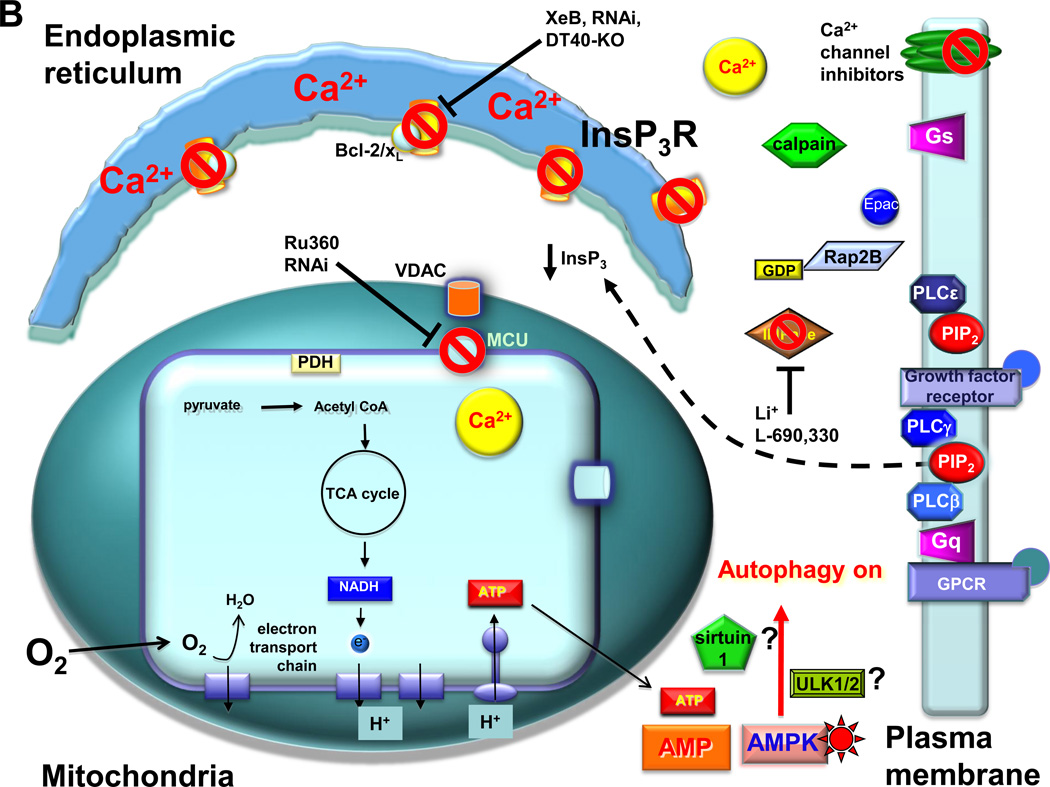
Fig. 2.
Suppression of InsP3R Ca2+ transfer to mitochondria induces mTOR-independent autophagy. A. In resting cells, low-level InsP3 production is maintained by circulating ligands for receptors that couple to phospholipases C (PLC) beta and gamma. In addition, InsP3R-mediated Ca2+ release and/or extracellular influx the Ca2+ can activate calpain, which cleaves and activates the heterotrimeric G-protein alpha subunit Gs, increasing the levels of cAMP, activating Epac to stimulate the small G protein Rap2B that activates PLCε. Phosphatidylinositol 4–5-biphosphate (PIP2) levels are maintained by a constant supply of inositol (Ins) by the inositol monophosphatase (IMPase). Low-level stochastic Ca2+ release from the endoplasmic reticulum by InsP3R, enhanced by Bcl-2 and Bcl-xL bound to the channel, provides Ca2+ to mitochondria in close proximity, mediated by the outer membrane VDAC and MCU at the inner mitochondrial membrane. Matrix Ca2+ activates pathways, including dehydrogenases and the F1-F0-ATPase, that fuel the electron transport chain and O2 consumption by providing reducing equivalents in the form of NADH. ATP production maintains a low cytoplasmic AMP:ATP ratio. This mechanism provides the cell with bioenergetic fitness that suppresses autophagy. B. The absence of constitutive delivery of Ca2+ from ER to mitochondria, due either to inhibition of InsP3R-mediated Ca2+ release (by genetic deletion of InsP3R (DT40-KO), inhibition of InsP3R activity (by xestospongin B (XeB), molecular ablation of the InsP3R (by RNAi), inhibition of InsP3 production by blocking the IMPase (by lithium (Li+) or L-690,330), blocking PLC (not shown; [37]), or inhibiting the calpain cascade that impinges on PLCε)); or by inhibition of mitochondrial Ca2+ uptake (Ru360 or MCU RNAi), results in diminished pyruvate dehydrogenase (PDH) activity (as well as the activities possibly other Ca2+-sensitive dehydrogenases and the ATP-synthase) that results in insufficient production of NADH, limiting the activity of the electron transport chain, diminishing O2 consumption and reducing ATP production. The consequent rise of the AMP:ATP ratio activates AMPK, which induces pro-survival, mTOR-independent autophagy, possibly involving ULK1/2 and/or sirtuin1.