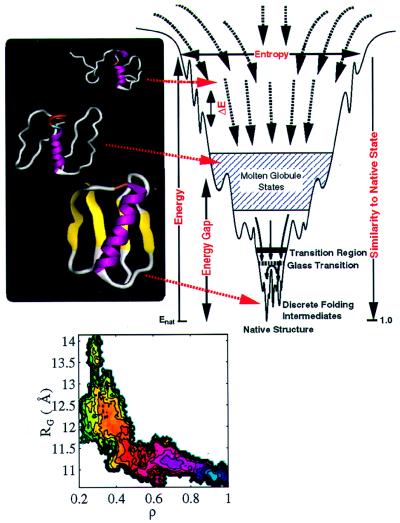
Figure 1.
The energy landscape for a folding protein. The major phenomonological parameters needed to capture this landscape include: the width of the funnel at small values of native similarity, indicating the entropy of denatured states; the roughness of the landscape, ΔE, which is related to the glass transition temperature, Tg; the stability of the native state relative to the collapsed but non-native (molten) globule states, the energy gap. The ribbon diagrams of the α/β protein, segment B1 of streptococcal protein G (GB1) provide structures from ensembles of unfolded, molten globule, and native conformations. The folding landscape for GB1 is projected onto two coordinates, the radius of gyration, Rg, of the folding globule, and the fraction of native contacts, ρ, which indicates how close the folding protein is to the native. The free energy change as folding occurs is shown as a contoured surface: (native) state corresponds to the blue region and the most unfavorable unfolded state is represented by the green contours.