Figure 2.
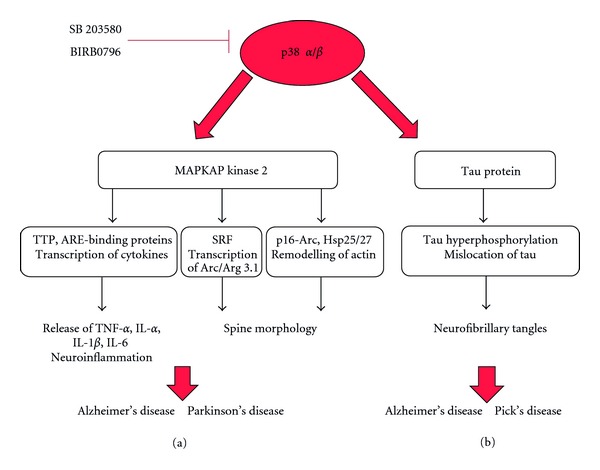
Schematic drawing illustrating the steps linking the p38 MAPK substrates to neurodegenerative disease. (a) The p38 MAPK-MK2 complex plays a role in neuroinflammation by phosphorylating AU-rich-element- (ARE-) binding proteins, such as tristetraprolin (TTP), which consequently can bind directly or indirectly to ARE sites present in TNF and other cytokine genes leading to transcription, translation, and subsequent release of mediators causing inflammation. The p38 MAPK-MK2 axis potentially plays an important role controlling dendritic spine morphology via direct activation of p16-Arc and Hsp, which are proteins involved in actin remodelling. Activity-dependent induction of p38 MAPK-MK2 axis can play an important role in the expression of the immediate early gene Arc/Arg3.1 which regulates spine morphology in neurons via activation of serum-response-factor- (SRF-) serum response element (SRE) complex. p38 MAPK-MK2 signalling cascade activation can have an effect on morphological changes observed at dendritic spines, a pattern that is observed during the development of neurodegenerative disease. (b) p38 MAPK phosphorylates tau protein at several residues. Hyperphosphorylated tau, contributes to the formation of tau oligomers. The aggregation of the tau oligomers forms the paired-helical filaments (PHFs), which then assemble together to form neurofibrillary tangles that are characteristically observed in the brain of patients suffering from Alzheimer's disease.
