Abstract
Introduction
Better understanding of aberrantly active molecular pathways in tumors offers potential to develop more specific and less toxic therapies. Abnormal mammalian target of rapamycin (mTOR) complex signaling and defects in TSC1 and TSC2 have been associated with the development of sub-ependymal giant cell astrocytomas (SEGAs) in tuberous sclerosis complex (TSC) patients. Recently, mTOR inhibitors such as everolimus have shown encouraging benefit for patients with SEGAs.
Areas covered
The authors discuss a molecular genetic pathway linked with TSC, specifically the role of two proteins whose functional absence is responsible for most SEGA tumors that arise in TSC patients. The authors also examine the rationale for targeted agents against this pathway therapeutically and describe the clinical evidence underlying the FDA approval of everolimus for patients with inoperable SEGAs.
Expert opinion
Everolimus (Afinitor®) selectively targets a molecular defect of SEGAs in TSC patients. Although surgery is effective, most SEGAs recur. An agent that inhibits an underlying molecular abnormality represents a particularly attractive therapeutic option for patients with inoperable or recurrent tumors. Studies are also underway to assess everolimus in treating other sequelae of TSC, and other gliomas. Finally, additional research aimed at better understanding aberrant cell signaling pathways may lead to the development of more effective therapeutics.
Keywords: astrocytoma, everolimus, low-grade glioma, mTOR inhibitor, tuberous sclerosis
1. Tuberous sclerosis
The tuberous sclerosis complex (TSC) is an autosomal dominant disorder with variable penetrance characterized by benign hamartomas in multiple organ systems including the brain, skin, kidney, lung, heart and retina [1]. The disease has a prevalence of 1 in 5800 live births with variable neurologic manifestations including mental retardation, autism and seizures due to various lesions in the CNS. Tubers, the most common lesions, are dysplastic foci thought to result from abnormal cell migration during embryogenesis and, though static, their numbers and locations are believed to correlate directly with the severity of neurologic symptoms.
Subependymal nodules (SENs), which are typically asymptomatic but can extend into the ventricles, are present in 88 - 95% of patients with TSC [2,3]. Subependymal giant cell astrocytomas (SEGAs) are thought to arise from SENs which enlarge causing symptoms, typically hydrocephalus. By following them radiographically, Growth rates of 1 mm/year to 1 mm/month have been observed based on serial MRI evaluations [4]. Histologically, giant cells that are polygonal, epithelioid or spindle-shaped are found in both SENs and SEGAs. SEGAs are benign tumorswith a low mitotic index, but vascular proliferation and necrosis may be seen. They are slow-growing, glioneuronal tumors arising near the foramen of Monro and develop in 5 - 20% of patients with TSC. SEGAs do not regress, and progressive growth is associated with an increased risk of illness and death, including sudden death from acute hydrocephalus [5,6].
The diagnostic triad of facial angiofibromas, mental retardation and seizures is present in fewer than half of patients with TSC and has been replaced by new diagnostic criteria involving both major and minor features, the presence of which allows a patient to be categorized as definitive, probable or possible TSC [7]. Treatment consists of surgical resection, when possible, but the risk of recurrence after incomplete resection is high, necessitating multiple resections. Recent efforts to better understand the molecular and genetic processes leading to TSC have determined that more than 85% of patients with TSC have inactivating mutations of TSC1 or TSC2 [8].
The TSC1 gene on chromosome 9q34, encodes a hydrophilic protein, hamartin [9], while the TSC2 gene on chromosome 16p13, encodes a 200-kDa protein, tuberin [10]. The TSC1 and TSC2 proteins form a heterodimeric complex, which regulates the mammalian target of rapamycin (mTOR) complex 1. The mTOR complex 1 is a serine/threonine kinase that regulates cell growth and proliferation in response to energy supply, stress and hypoxia by interacting with a number of transcription factors [11]. Tuberin contains a GTPase activating protein (GAP) domain that has been shown to stimulate the Ras homolog enriched in brain (RHEB) GTP-binding protein [12], which in turn activates mTOR complex 1. TSC1 interacts directly with TSC2 and is believed to protect it from degradation [13]. In addition, TSC2 has its own transcription activation domain whose activity is modulated by members of the steroid receptor superfamily [14]. The proteins encoded by TSC1 and TSC2 are part of a tumor-suppressor complex. When either TSC1 or TSC2 is deficient, mTOR complex 1 is constitutively activated, leading to abnormal cellular growth, proliferation and protein synthesis [15,16]. Both sirolimus and everolimus effectively inhibit mTOR complex 1, correcting the molecular defect of the tuberous sclerosis complex [17]. In addition, regression of SEGAs has been reported among patients treated with either sirolimus or everolimus [18,19].
2. Sirolimus
Sirolimus, also known as rapamycin (Rapamune; Pfizer, Inc. New York, USA) is an immunosuppressant that binds to the - FK-binding protein 12 (FKBP12). The sirolimus-FKBP12 complex binds directly to mTOR complex 1, thereby blocking mTOR phosphorylation and downstream signaling [20]. Sirolimus has been shown to inhibit the growth of TSC-deficient cells in vitro and to downregulate mTOR activity in renal tumors of Eker rats that carry a germline TSC2 mutation [21].
Angiomyolipomas in patients with TSC regressed when treated with sirolimus administered to achieve serum concentrations of 1 - 5 ng/ml, and increased in volume upon cessation of therapy. Cerebral lesions were unchanged however [22]. Koenig et al. reported the case of a 21-year-old woman with TSC receiving sirolimus (serum concentrations 11 - 13 ng/ml) for bilateral SEGAs. After 2.5 months of treatment, both tumors decreased in size [19]. Franz et al. reported the outcome of four TSC patients with SEGAs who were treated with oral sirolimus at standard immunosuppressive doses (serum concentrations 5 - 15 ng/ml). The treatment was well tolerated and all lesions showed regression, which included necrosis in one case. Upon interruption of therapy, a SEGA regrew in one patient, but then regressed upon therapy resumption [18].
3. Everolimus
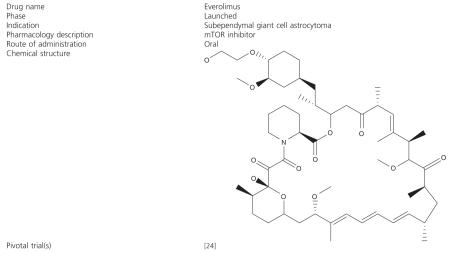
Everolimus (Box 1), marketed by Novartis (Basel, Switzerland) under the trade name Afinitor®, is the 40-O-(2-hydroxyethyl) derivative of sirolimus and works similarly to sirolimus as an mTOR inhibitor [23]. Everolimus is FDA-approved for the treatment of renal cancer and for organ rejection prophylaxis, and, as of October 2010, Phase III trials are under way in breast cancer, gastric cancer, hepatocellular carcinoma, pancreatic neuroendocrine tumors and lymphoma.
Box 1. Drug summary.
Pharmaprojects - copyright to Citeline Drug Intelligence (an Informa business). Readers are referred to Pipeline (http://informa-pipeline.citeline.com) and Citeline (http://informa.citeline.com).
A recent Phase II trial reported the effectiveness of everolimus in treating SEGAs associated with tuberous sclerosis [24]. Twenty-eight patients were enrolled in the study and ranged in age from 3 to 34 years old. All had a diagnosis of TSC and documentation of serial growth (on two successive MRI scans) of a SEGA. The primary end point was the change in volume of the SEGA during 6 months’ treatment, while seizure frequency, neuro-cognition and quality of life were secondary end points. The dose of everolimus was adjusted to achieve a trough concentration of 5 - 15 ng/ml (median 5 ng/ml at 6 months). Twenty-seven patients completed the core 6-month treatment phase and continued therapy. Tumor volume was reduced by at least 30% during the 6-month treatment phase in 75% of patients (p < 0.001), while nine patients (32%) had reductions of 50% or more. Everolimus therapy was also associated with a clinically relevant reduction in the overall frequency of clinical and subclinical seizure (median change, -1 seizure; p = 0.02). Overall scores on the Quality of Life in Childhood Epilepsy measure also improved from a mean (± SD) of 57.8 ± 14 at baseline to 63.4 ± 12.4 and 62.1 ± 14.2 at 3 and 6 months, respectively. No patient required surgery or developed new SEGAs while receiving everolimus. Additionally, one patient who met the criteria for treatment success stopped therapy but restarted treatment when regrowth of the tumor became evident. Overall, therapy with everolimus on this study was well tolerated. Most adverse events were mild and included upper respiratory tract, ear and sinus infections, stomatitis, fever, transaminase elevation and hyperlipidemia. Based on the encouraging results observed on this study, on 29 October 2010 the FDA granted accelerated approval of everolimus for SEGAs that are not candidates for surgical resection.
4. Conclusion
The molecular basis for SEGA formation in patients with TSC has become clearer with our understanding of the role of the TSC1 and TSC2 gene products and their role in mTOR signaling. Also, pharmacologic agents that inhibit this pathway have enabled us to target precisely the defect in patients with TSC. Sirolimus has shown activity in the treatment of SEGAs, while the noteworthy activity of everolimus among TSC patients with SEGAs in the recent Phase II study described above has led to accelerated FDA approval of everolimus for SEGAs that are not resectable. A Phase III, randomized, placebo-controlled study has recently been initiated with a primary end point of radiographic response of SEGA tumors (www.clinicaltrials.gov identifier: NCT00789828). Secondary end points of this study include time to progression as well as decrease in seizure frequency.
Owing to the encouraging response of angiomyolipomas and lymphangioleiomyomatomas to the mTOR inhibitor sirolimus in an earlier study [22], a randomized, double-blind, placebo-controlled Phase III trial (www.clinicaltrials.gov identifier: NCT00790400) is now underway to evaluated everolimus in the treatment of angiomyolipomas among patients with either TSC or sporadic lymphangioleiomyomatosis (LAM). The primary end point of this study is radiographic response, while secondary end points include time to progression, skin lesion regression and changes in pulmonary function (LAM patients only).
Finally, everolimus is also being evaluated against other primary CNS tumors. A Phase II study is underway among adults with recurrent or progressive low-grade glioma (www.clinicaltrials.gov identifier: NCT00831324). Clinical studies of mTOR inhibitors have demonstrated limited activity among recurrent glioblastoma patients [25-28]. One possibility limiting the activity of mTOR inhibitors that does not seem to occur in SEGAs is loss of negative feedback and induction of other pathways including the mitogen-activated protein kinase cascade [29]. A Phase II study is evaluating the addition of everolimus to standard temozolomide chemoradiotherapy for newly diagnosed adults with glioblastoma (www.clinicaltrials.gov identifier: NCT00553150).
Though the ideal dose and length of treatment are not fully understood at present, as the molecular mechanisms leading to the development of SEGAs and other primary brain tumors are better understood, our ability successfully to treat these neoplasms will inevitably improve as new and more specific molecular targets emerge.
5. Expert opinion
Oncology has benefited greatly from research aimed at improving understanding of dysregulated cell signaling pathways. As the molecular pathways associated with tumorigenesis are better understood, more effective therapeutic agents can be developed with greater specificity, while, it is hoped, minimizing side effects that commonly plague patients undergoing chemotherapy. The molecular defect responsible for the development of subependymal giant cell astrocytomas (SEGAs) in patients with the tuberous sclerosis complex (TSC) has been elucidated and two gene products have been identified. Several therapeutic agents that correct this defect are now being studied including everolimus, a potent inhibitor of the mTOR signaling pathway.
Primary CNS tumors are rare, and our understanding of the mechanisms leading to their formation is incomplete, allowing us to target only a few molecules so far. However, genetic analysis and patient outcome studies have allowed us to distinguish and reclassify various tumor subtypes based not only on histology but on genetic and molecular criteria [30,31]. The description of subgroups within tumor types can also help predict response to therapy and possibly the likelihood of side effects from therapeutic agents. Two genes involved in the mTOR signaling pathway, when mutated, lead to SEGA formation; but 15% of patients with TSC have mutations in neither of these genes, reinforcing the incomplete nature of our understanding. However, as we better understand these diseases, we shall discover new pathways and new targets, with an ultimate goal of being able to design therapy for individual patients based on the mutations that are specific to their respective tumor. Not only should this approach be more successful in treating the tumor, but we should also be able to reduce side effects based on systemic exposures and medication interactions. The goal of customdesigned therapy is achievable as our understanding and technological skill increase.
In addition to chemotherapeutic agents and radiation, new categories of antitumor agents are being developed, the most prominent of which for treating brain tumors is bevacizumab, a monoclonal antibody directed against vascular endothelial growth factor. Additional antiangiogenic treatments are also being evaluated for CNS tumor patients. Like targets in the mTOR pathway, as new molecules are identified in angiogenic pathways, new targets emerge and should lead to exciting new treatments. Ultimately, by combining multiple agents targeting separate pathways in a way specific to each tumor’s genetic profile, we should be able more effectively to impact the tumor directly and potentially limit the emergence of resistance.
Footnotes
Declaration of interest
None of the authors of this manuscript has received financial compensation for the preparation of this manuscript. SG Turner, KB Peters and A Desjardins state no conflict of interest. JJ Vredenburgh has received financial compensation as a consultant for Merck/Schering-Plough and Roche/Genentech and is also a member of the speakers’ bureau for Merck/Schering-Plough and Roche/Genentech. DA Reardon has received financial compensation as a consultant for Merck/Schering-Plough, Roche/Genentech and Merck KGaA/EMD Serono and is also a member of the speakers’ bureau for Merck/Schering-Plough and Roche/Genentech. HS Friedman has served on an advisory board for Roche/Genentech and is a member of the speakers’ bureau.
Bibliography
- 1.Krueger DA, Franz DN. Current management of tuberous sclerosis complex. Paediatr Drugs. 2008;10:299–313. doi: 10.2165/00148581-200810050-00004. [DOI] [PubMed] [Google Scholar]
- 2.Braffman BH, Bilaniuk LT, Naidich TP, et al. MR imaging of tuberous sclerosis: pathogenesis of this phakomatosis, use of gadopentetate dimeglumine, and literature review. Radiology. 1992;183:227–38. doi: 10.1148/radiology.183.1.1549677. [DOI] [PubMed] [Google Scholar]
- 3.Nabbout R, Santos M, Rolland Y, et al. Early diagnosis of subependymal giant cell astrocytoma in children with tuberous sclerosis. J Neurol Neurosurg Psychiatry. 1999;66:370–5. doi: 10.1136/jnnp.66.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuccia V, Zuccaro G, Sosa F, et al. Subependymal giant cell astrocytoma in children with tuberous sclerosis. Childs Nerv Syst. 2003;19:232–43. doi: 10.1007/s00381-002-0700-2. [DOI] [PubMed] [Google Scholar]
- 5.Adriaensen ME, Schaefer-Prokop CM, Stijnen T, et al. Prevalence of subependymal giant cell tumors in patients with tuberous sclerosis and a review of the literature. Eur J Neurol. 2009;16:691–6. doi: 10.1111/j.1468-1331.2009.02567.x. [DOI] [PubMed] [Google Scholar]
- 6.Franz DN, de Vries PJ, Crino PB. Giant cell astrocytomas in tuberous sclerosis complex. Arch Dis Child. 2009;94:75–6. [PubMed] [Google Scholar]
- 7.Roach ES, Gomez MR, Northrup H. Tuberous sclerosis complex consensus conference: revised clinical diagnostic criteria. J Child Neurol. 1998;13:624–8. doi: 10.1177/088307389801301206. [DOI] [PubMed] [Google Scholar]
- 8.Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355:1345–56. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- 9.van Slegtenhorst M, de Hoogt R, Hermans C, et al. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science. 1997;277:805–8. doi: 10.1126/science.277.5327.805. [DOI] [PubMed] [Google Scholar]
- 10.The European Chromosome 16 Tuberous Sclerosis Consortium Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell. 1993;75:1305–15. doi: 10.1016/0092-8674(93)90618-z. [DOI] [PubMed] [Google Scholar]
- 11.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–45. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 12.Inoki K, Li Y, Xu T, et al. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003;17:1829–34. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benvenuto G, Li S, Brown SJ, et al. The tuberous sclerosis-1 (TSC1) gene product hamartin suppresses cell growth and augments the expression of the TSC2 product tuberin by inhibiting its ubiquitination. Oncogene. 2000;19:6306–16. doi: 10.1038/sj.onc.1204009. [DOI] [PubMed] [Google Scholar]
- 14.Henry KW, Yuan X, Koszewski NJ, et al. Tuberous sclerosis gene 2 product modulates transcription mediated by steroid hormone receptor family members. J Biol Chem. 1998;273:20535–9. doi: 10.1074/jbc.273.32.20535. [DOI] [PubMed] [Google Scholar]
- 15.Chan JA, Zhang H, Roberts PS, et al. Pathogenesis of tuberous sclerosis subependymal giant cell astrocytomas: biallelic inactivation of TSC1 or TSC2 leads to mTOR activation. J Neuropathol Exp Neurol. 2004;63:1236–42. doi: 10.1093/jnen/63.12.1236. [DOI] [PubMed] [Google Scholar]
- 16.Huang J, Manning BD. The TSC1-TSC2 complex: a molecular switchboard controlling cell growth. Biochem J. 2008;412:179–90. doi: 10.1042/BJ20080281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Easton JB, Houghton PJ. mTOR and cancer therapy. Oncogene. 2006;25:6436–46. doi: 10.1038/sj.onc.1209886. [DOI] [PubMed] [Google Scholar]
- 18.Franz DN, Leonard J, Tudor C, et al. Rapamycin causes regression of astrocytomas in tuberous sclerosis complex. Ann Neurol. 2006;59:490–8. doi: 10.1002/ana.20784. [DOI] [PubMed] [Google Scholar]
- 19.Koenig MK, Butler IJ, Northrup H. Regression of subependymal giant cell astrocytoma with rapamycin in tuberous sclerosis complex. J Child Neurol. 2008;23:1238–9. doi: 10.1177/0883073808321764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plas DR, Thomas G. Tubers and tumors: rapamycin therapy for benign and malignant tumors. Curr Opin Cell Biol. 2009;21:230–6. doi: 10.1016/j.ceb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 21.Kenerson H, Dundon TA, Yeung RS. Effects of rapamycin in the Eker rat model of tuberous sclerosis complex. Pediatr Res. 2005;57:67–75. doi: 10.1203/01.PDR.0000147727.78571.07. [DOI] [PubMed] [Google Scholar]
- 22.Bissler JJ, McCormack FX, Young LR, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. 2008;358:140–51. doi: 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Houghton PJ. Everolimus. Clin Cancer Res. 2010;16:1368–72. doi: 10.1158/1078-0432.CCR-09-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krueger DA, Care MM, Holland K, et al. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med. 2010;363:1801–11. doi: 10.1056/NEJMoa1001671. [DOI] [PubMed] [Google Scholar]
- 25.Chang SM, Kuhn J, Wen P, et al. Phase I/pharmacokinetic study of CCI-779 in patients with recurrent malignant glioma on enzyme-inducing antiepileptic drugs. Investigational New Drugs. 2004;22:427–35. doi: 10.1023/B:DRUG.0000036685.72140.03. [DOI] [PubMed] [Google Scholar]
- 26.Chang SM, Wen P, Cloughesy T, et al. Phase II study of CCI-779 in patients with recurrent glioblastoma multiforme. Investigational New Drugs. 2005;23:357–61. doi: 10.1007/s10637-005-1444-0. [DOI] [PubMed] [Google Scholar]
- 27.Galanis E, Buckner JC, Maurer MJ, et al. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: A North Central Cancer Treatment Group Study. J Clin Oncol. 2005;23:5294–304. doi: 10.1200/JCO.2005.23.622. [DOI] [PubMed] [Google Scholar]
- 28.Cloughesy TF, Yoshimoto K, Nghiemphu P, et al. Antitumor activity of rapamycin in a phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLOS Medicine V. 2008 Jan 22;5(1):e8. doi: 10.1371/journal.pmed.0050008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carracedo A, Ma L, Teruya-Feldstein J, et al. Inhibition of MTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118:3065–74. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niyazi M, Siefert A, Schwarz SB, et al. Therapeutic options for recurrent malignant glioma. Radiother Oncol. 2011;98:1–14. doi: 10.1016/j.radonc.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Van Meir EG, Hadjipanayis CG, Norden AD, et al. Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin. 2010;60:166–93. doi: 10.3322/caac.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]