Abstract
Anti-inflammatory effects of Houttuynia cordata supercritical extract (HSE) were investigated in rat carrageenan-air pouch model. Oral administration of HSE (50-200 mg/kg) suppressed carrageenan-induced exudation and albumin leakage, as well as inflammatory cell infiltration at a high dose (200 mg/kg). Intraperitoneal injection of dexamethasone (2 mg/kg) only decreased exudation and cell infiltration, while indomethacin (2 mg/kg, i.p.) reduced exudate volume and albumin content without influence on the cell number. HSE lowered tumor-necrosis factor-α (TNF-α) and nitric oxide (NO), as well as prostaglandin E2 (PGE2). Dexamethasone only reduced TNF-α and NO, while indomethacin decreased PGE2. The results indicate that HSE exhibits anti-inflammatory effects by inhibiting both TNF-α-NO and cyclooxygenase-2-PGE2 pathways.
Keywords: Carrageenan, inflammation, Houttuynia cordata, supercritical extract
It has been reported that Houttuynia (H.) cordata extracts have diverse pharmacological effects including antiviral and antibacterial [1,2], antiallergic [3,4], antioxidant [5,6] and antimutagenic activities [5]. Thus, H. cordata Thunb has been used as an Oriental medicine for the therapy of inflammatory diseases such as ulcerative colitis [7].
Recent studies revealed that the constituents of H. cordata essential oil are methyl nonyl ketone, β-myrcene, β-pinene, α-pinene, α-terpineol and n-decanoic acid, and that the anti-inflammatory effects of this oil were also demonstrated [8]. Notably, the essential oil constituents and their concentrations varied with different extraction methods [2]. Recently, supercritical extraction technology has been adopted for the total extraction of constituents from natural plant sources.
In a previous study, we demonstrated the anti-inflammatory activity of H. cordata supercritical extract (HSE) in both macrophage cell line and a mouse model of carrageenan-induced air pouch inflammation [9], an in vivo model suitable for the analysis of diverse biochemical and pathological parameters [10,11]. In the present study, we evaluated the effectiveness of HSE in a rat model of air pouch inflammation, since the change in the inflammatory parameters was found to be different in mouse and rat models.
The aerial part of H. cordata was extracted for 2 hours under CO2 supercritical conditions (temperature 60℃, pressure 400 bar). After separating the CO2 solvent by reduced pressure, the extract (yield=1.5%) was collected [12]. The extract was dissolved in soybean oil, and orally administered at 4 mL/kg.
Six-week-old male Sprague-Dawley rats (body weight 200-220 g; n=8/group) (Orient-Bio, Seongnam, Korea) were subcutaneously injected with 20 mL of sterile air into the back side to form a pouch [10,11]. After 2 and 5 days, the pouch was re-injected with 10 mL of air. Twenty four hours after the final air injection, HSE (20, 65 or 200 mg/kg) was orally administered, followed 30 min later by injection with 2 mL of lambda carrageenan (1% in saline; Sigma-Aldrich, St. Louis, USA) into the pouch. For comparisons, additional rats were given intraperitoneal injections of either dexamethasone (2 mg/kg; Sigma) or indomethacin (2 mg/kg; Sigma). The animal experiments were approved by the Institutional Animal Care and Use Committee of the Laboratory Animal Research Center, Chungbuk National University, Korea.
The pouch was washed with 2 mL of cold saline after 6 hours, and the net volume of lavage fluid was recorded. Total numbers of inflammatory cells and albumin, a marker of vascular leakage, were determined using a coulter counter and a blood biochemistry analyzer, respectively. Tumor-necrosis factor-α (TNF-α) and interleukin-6 (IL-6) were analyzed using enzyme-linked immunosorbent assay (ELISA) kits (Komabiotech, Seoul, Korea). The concentrations of NO and PGE2 were determined by Griess reagent (Sigma) and enzyme immunoassay (EIA) using a Correlate-EIA kit (Assay Designs, Ann Arbor, Ann ArboUSA), respectively. The results were expressed as the mean±SD. Tests of significance were performed using Duncan's multiple-range test after one-way analysis of variance, with P<0.05 as a criterion of difference.
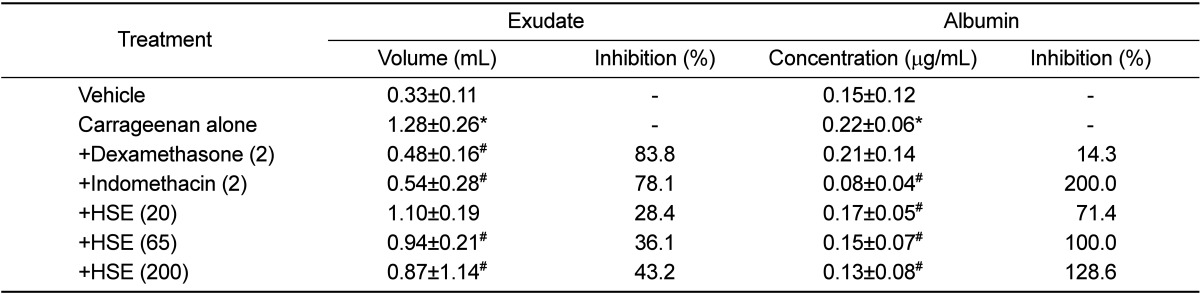
Injection of carrageenan into rat air pouches significantly increased the exudate volume in the pouches and the albumin contents in the exudate (Table 1). However, oral treatment with HSE suppressed the carrageenan-induced increases in both the exudate volume and albumin leakage in a dose-dependent manner, inhibiting by 28.4-43.2% and 71.4-128.6% at 20-200 mg/kg, respectively. For comparison, intraperitoneal administration of indomethacin (2 mg/kg) also reduced both the exudate volume (78.1%) and albumin content (200%). In contrast, dexamethasone (2mg/kg) specifically suppressed exudates volume (83.8%), but not albumin leakage (14.3%).
Table 1.
Effects of dexamethasone, indomethacin and Houttuynia cordata supercritical extract on carrageenan-induced exudation and inflammatory cell infiltration in rat air pouches (n=8)
HSE: Houttuynia cordata supercritical extract. *Significantly different from vehicle control, P<0.05. #Significantly different from carrageenan alone, P<0.05.
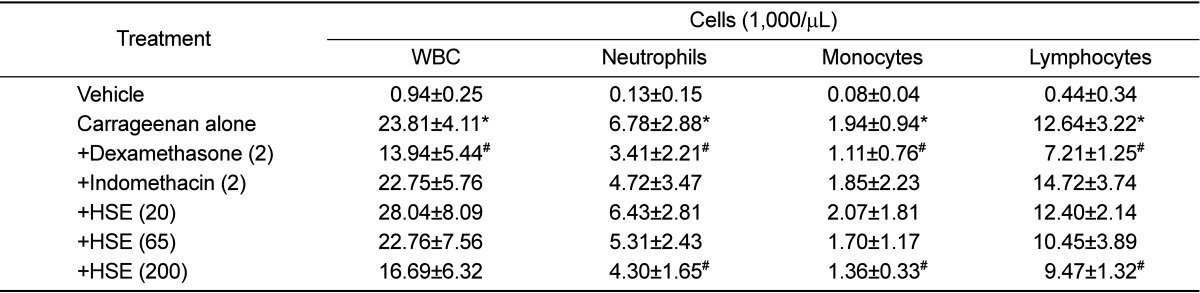
Total white blood cells (WBC) in the exudate, including neutrophils, monocytes and lymphocytes, were greatly increased by carrageenan (Table 2). Interestingly, infiltrating inflammatory cells were suppressed to by a high dose of HSE (200 mg/kg) and dexamethasone, but not by indomethacin.
Table 2.
Effects of dexamethasone, indomethacin and Houttuynia cordata supercritical extract on carrageenan-induced exudation and inflammatory cell infiltration in rat air pouches (n=8)
HSE: Houttuynia cordata supercritical extract, WBC: white blood cells. *Significantly different from vehicle control, P<0.05. #Significantly different from carrageenan alone, P<0.05.
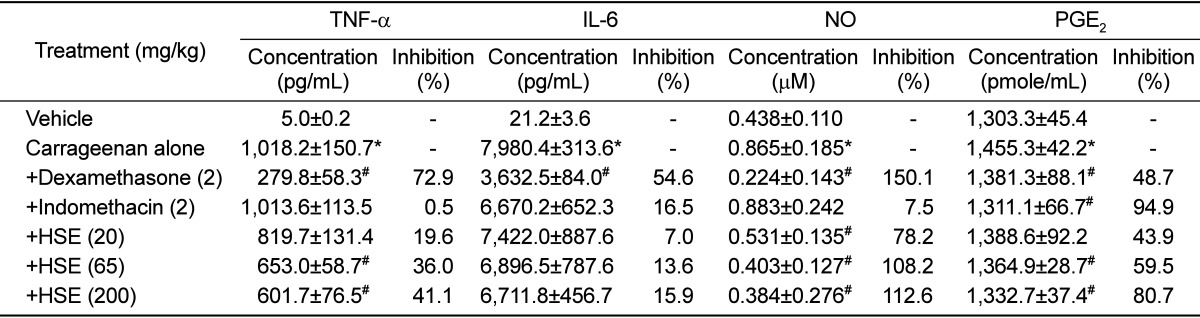
Carrageenan enormously enhanced major inflammatory cytokines TNF-α and IL-6 in the exudate (Table 3). Administration of HSE (65-200mg/kg) and dexamethasone, significantly lowered the carrageenan-induced increases in TNF-α, while indomethacin was ineffective. In comparison, the increased IL-6 level was significantly attenuated only by dexamethasone. Interestingly, HSE (65-200mg/kg) markedly blocked the carrageenan-induced increases in both NO and PGE2. Dexamethasone specifically inhibited NO production, while indomethacin reversed PGE2 concentration to the control level.
Table 3.
Effects of dexamethasone, indomethacin and Houttuynia cordata supercritical extract on the carrageenan-induced increases in cytokines and inflammatory mediators in rat air pouch exudates (n=8)
HSE: Houttuynia cordata supercritical extract, TNF-α: tumor-necrosis factor-α, IL-6: interleukin-6, NO: nitric oxide, PGE2: prostaglandin E2. *Significantly different from vehicle control, P<0.05. #Significantly different from carrageenan alone, P<0.05.
In comparison with the mouse air-pouch model [9], higher and lower responses to carrageenan exposure in exudate volume and albumin leakage, respectively, were observed in the rat model. That is, 1.80- and 3.88-fold increases in exudate volumes and 2.64- and 1.47-fold increases in albumin contents in mice and rats, respectively, were observed following carrageenan injection. An extract of H. cordata obtained under a supercritical condition markedly attenuated the secretion of both major inflammatory mediators, NO and PGE2. The numbers of neutrophils, monocytes and lymphocytes increased by carrageenan were reduced following treatment with HSE (200 mg/kg). This was an effect obtained by dexamethasone, but not by indomethacin (1-2 mg/kg) [11,13]. Although migration of monocytes was suppressed only by a high dose (200 mg/kg) of HSE, the corticosteroid-like effect of HSE was confirmed by its inhibitory action on TNF-α and NO, which are the main inflammatory mediators from macrophages. Therefore, the effect of HSE on macrophages may come from mainly the suppression of signaling pathways, as supported by the highly-sensitive inhibition by HSE of NO secretion from RAW 264.7 cells [9].
In addition to the TNF-α-NO pathway, HSE exerted inhibitory activity on the in vivo release of PGE2, which is produced from arachidonic acid via cyclooxygenase-2 (COX-2). It is assumed that HSE directly inhibits COX-2 or deactivates inflammatory cells expressing COX-2 [9]. Although there was a different sensitivity to NO and PGE2, the production of PGE2 was also markedly suppressed by HSE treatment in RAW 264.7 cells, mice and rats [9, the present study]. Such a difference between in vitro and in vivo or between mice and rats studies may be due to the different animals and stimulators, i.e., lipopolysaccharide (LPS) and carrageenan.
There are few reports demonstrating the anti-inflammatory effects of H. cordata. Intravenous injection of H. cordata essential oils (including 22 constituents) reduced inflammatory responses in a carrageenan-induced pleurisy model, and attenuated xylene-induced ear edema [8]. Notably, our previous result shows that supercritical extract of H. cordata (IC50<0.001%) is much superior to an aqueous extract (IC50≒0.1%) in the inhibition of NO production by RAW 264.7 cells [9,14].
Notably, supercritical extraction technology has been adopted for the total extraction of constituents from natural plant sources. We assessed the anti-inflammatory effects of HSE by analyzing mediators in the two major pathways of inflammation, TNF-α-NO and COX-2-PGE2, in comparison with the synthetic steroid dexamethasone and a non-steroidal anti-inflammatory drug (NSAID) indomethacin. We demonstrated dual actions of HSE in the inflammatory process, dexamethasone- and indomethacin-like effects. Therefore, a supercritical extract of H. cordata could be a good drug candidate for the relief of various types of inflammation responsive to corticosteroids or NSAIDs.
Acknowledgments
This work was supported by Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011-0031403).
References
- 1.Hayashi K, Kamiya M, Hayashi T. Virucidal effects of the steam distillate from Houttuynia cordata and its components on HSV-1, influenza virus, and HIV. Planta Med. 1995;61(3):237–241. doi: 10.1055/s-2006-958063. [DOI] [PubMed] [Google Scholar]
- 2.Lu H, Wu X, Liang Y, Zhang J. Variation in chemical composition and antibacterial activities of essential oils from two species of Houttuynia THUNB. Chem Pharm Bull (Tokyo) 2006;54(7):936–940. doi: 10.1248/cpb.54.936. [DOI] [PubMed] [Google Scholar]
- 3.Lee JS, Kim IS, Kim JH, Kim JS, Kim DH, Yun CY. Suppressive effects of Houttuynia cordata Thunb (Saururaceae) extract on Th2 immune response. J Ethnopharmacol. 2008;117(1):34–40. doi: 10.1016/j.jep.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 4.Li GZ, Chai OH, Lee MS, Han EH, Kim HT, Song CH. Inhibitory effects of Houttuynia cordata water extracts on anaphylactic reaction and mast cell activation. Biol Pharm Bull. 2005;28(10):1864–1868. doi: 10.1248/bpb.28.1864. [DOI] [PubMed] [Google Scholar]
- 5.Chen YY, Liu JF, Chen CM, Chao PY, Chang TJ. A study of the antioxidative and antimutagenic effects of Houttuynia cordata Thunb. using an oxidized frying oil-fed model. J Nutr Sci Vitaminol (Tokyo) 2003;49(5):327–333. doi: 10.3177/jnsv.49.327. [DOI] [PubMed] [Google Scholar]
- 6.Toda S. Antioxidative effects of polyphenols in leaves of Houttuynia cordata on protein fragmentation by copper-hydrogen peroxide in vitro. J Med Food. 2005;8(2):266–268. doi: 10.1089/jmf.2005.8.266. [DOI] [PubMed] [Google Scholar]
- 7.Jiang XL, Cui HF. Different therapy for different types of ulcerative colitis in China. World J Gastroenterol. 2004;10(10):1513–1520. doi: 10.3748/wjg.v10.i10.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu HM, Liang YZ, Yi LZ, Wu XJ. Anti-inflammatory effect of Houttuynia cordata injection. J Ethnopharmacol. 2006;104(1-2):245–249. doi: 10.1016/j.jep.2005.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shin S, Joo SS, Jeon JH, Park D, Jang MJ, Kim TO, Kim HK, Hwang BY, Kim KY, Kim YB. Anti-inflammatory effects of a Houttuynia cordata supercritical extract. J Vet Sci. 2010;11(3):273–275. doi: 10.4142/jvs.2010.11.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romano M, Faggioni R, Sironi M, Sacco S, Echtenacher B, Di Santo E, Salmona M, Ghezzi P. Carrageenan-induced acute inflammation in the mouse air pouch synovial model. Role of tumour necrosis factor. Mediators Inflamm. 1997;6(1):32–38. doi: 10.1080/09629359791901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wallace JL, Chapman K, McKnight W. Limited anti-inflammatory efficacy of cyclo-oxygenase-2 inhibition in carrageenan-airpouch inflammation. Br J Pharmacol. 1999;126(5):1200–1204. doi: 10.1038/sj.bjp.0702420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee HY, Kim YJ, Kim EJ, Song YK, Byun SY. Red pigment from Lithospermum erythrorhizon by supercritical CO2 extraction. J Cosmet Sci. 2008;59(5):431–440. [PubMed] [Google Scholar]
- 13.Oliveira de Melo J, da Conceição Torrado Truiti M, Muscará MN, Bolonheis SM, Dantas JA, Caparroz-Assef SM, Cuman RK, Bersani-Amado CA. Anti-inflammatory activity of crude extract and fractions of Nectandra falcifolia leaves. Biol Pharm Bull. 2006;29(11):2241–2245. doi: 10.1248/bpb.29.2241. [DOI] [PubMed] [Google Scholar]
- 14.Park E, Kum S, Wang C, Park SY, Kim BS, Schuller-Levis G. Anti-inflammatory activity of herbal medicines: inhibition of nitric oxide production and tumor necrosis factor-α secretion in an activated macrophage-like cell line. Am J Chin Med. 2005;33(3):415–424. doi: 10.1142/S0192415X05003028. [DOI] [PubMed] [Google Scholar]