Figure 4. Chemical tools to study redox biology.
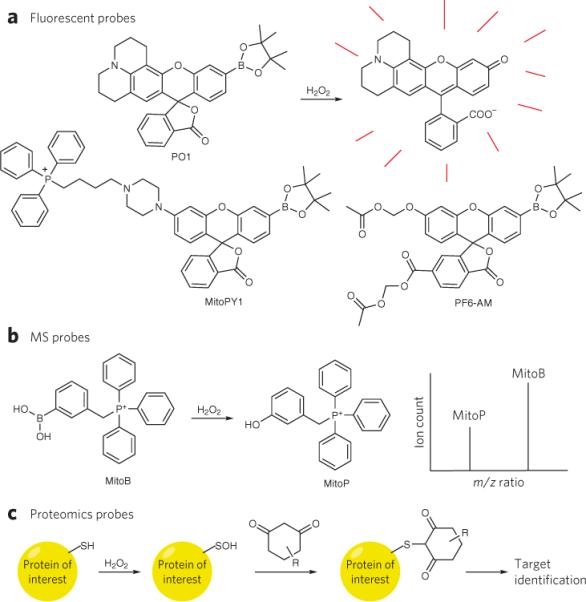
(a) The conversion of boronates to phenols by H2O2 has been used to create a suite of novel fluorescent probes with various properties, such as red-shifted emission (Peroxy Orange 1, PO1), mitochondrial localization (Mitochondria Peroxy Yellow 1, MitoPY1) and enhanced sensitivity through cytosolic trapping groups (Peroxyfluor-6 acetoxymethyl ester, PF6-AM). (b) A mitochondrial-targeted MS probe, which similarly uses the conversion of a boronic acid to a phenol, allows ratiometric detection and quantification of H2O2 in vivo by analysis of the ion count ratios between the protected and deprotected form of the probe, which can be distinguished by differences in mass to charge (m/z) ratios. (c) Dimedone-based reactivity probes can trap oxidized cysteine residues from a sulfenic acid and when coupled to purification or labeling groups, allow the identification of the redox-modified target.
