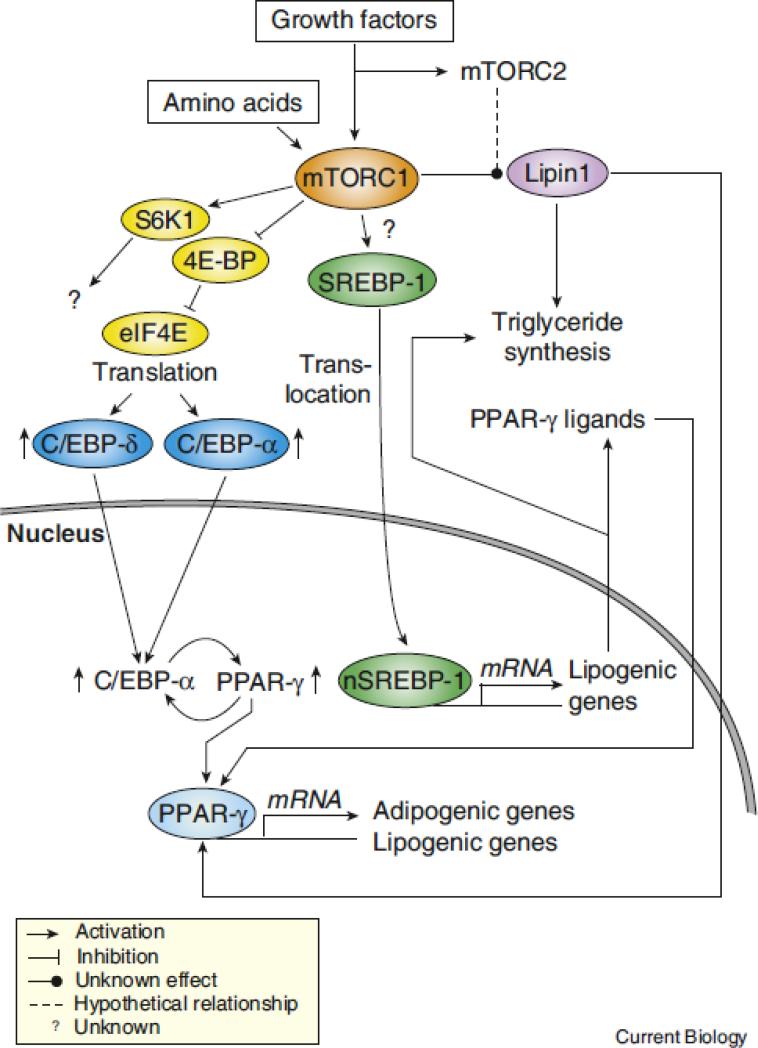
Figure 3. mTORC1 promotes adipogenesis and adipocyte maintenance through various mechanisms.
Many evidences indicates that mTORC1 controls adipogenesis by activating PPAR-γ, a nuclear receptor controlling the expression of numerous genes involved in fatty acid uptake, synthesis, esterification, and storage. Many mechanisms are though to be involved in the regulation of PPAR-γ by mTORC1. Activation of mTORC1 induces the phosphorylation of 4E-BPs, which in turn releases eIF4E and increases the translation of C/EBP-α and -δ, which are key components required for the establishment of the adipogenic cascade. C/EBP-δ is known to drive the expression of C/EBP-α and PPAR-γ and to trigger the activation of a feed-forward loop in which these two transcription factors reciprocally induce their expression. When sufficient levels of PPAR-γ proteins are produced, this transcription factor promotes adipogenesis and lipid synthesis by inducing the expression of many lipogenic genes. SREBP-1 cleavage by mTORC1 may also contribute to the induction of adipognesis by directly favoring triglyceride synthesis and by promoting the production of endogenous ligands for PPAR-γ. More work is required to determine if SREBP-1-dependent production of PPAR-γ ligands plays a significant role in the activation of PPAR-γ by mTORC1. Lipin1, a phosphatidic acid phosphatase, was shown to play key role in adipogenesis by promoting triglyceride synthesis and by serving as a coactivator for PPAR-γ. Lipin1 is phosphorylated in response to insulin and amino acids in a rapamycin-sensitive fashion. Because rapamycin can inhibit mTORC2 activity in some cell types, it is unclear if lipin1 is a direct substrate of mTORC1 or mTORC2. The biological meaning of mTOR-mediated lipin1 phosphorylation remains to be characterized.