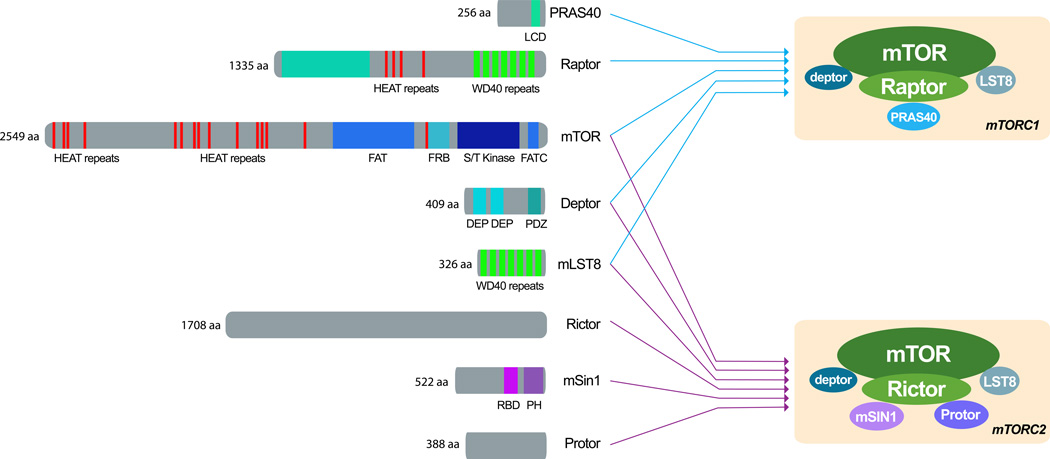
Figure 1. Domain organization of mTOR and mTOR-interacting proteins.
A. mTORC1 and mTORC2 have shared (mTOR, mLST8 and deptor) and unique components. Raptor and PRAS40 are unique to mTORC1 and rictor, mSin1 and Protor are specific to mTORC2. The domain organization of mTOR resembles that of other PIKK family members. At the N-terminus there is a cluster of Huntingtin, Elongation factor 3, a subunit of protein phosphatase 2A and TOR1 (HEAT) repeats, which mediate protein-protein interactions. These are followed by, a FRAP, ATM and TRRAP (FAT) domain, the FKBP12-Rapamycin Binding (FRB) domain (which mediates the inhibitory action of rapamycin), the serine/threonine kinase catalytic domain and the C-terminal FATC domain. mLST8 is highly conserved; its seven WD40 domains form a beta propeller that mediates protein-protein interactions. Deptor consists of a tandem dishevelled, egl-10, pleckstrin (DEP) domains, followed by a single postsynaptic density 95, discs large, zonula occludens-1 (PDZ) domain. The scaffolding function of raptor is reflected by its composition of protein-binding domains; it consists of several HEAT repeats, followed by seven WD40 motifs, probably arranged in a beta propeller.Rictor has no clearly identifiable domains or motifs despite its key role in mTORC2 function; likewise, Protor lacks identifiable domains or motifs, and its function awaits clarification.