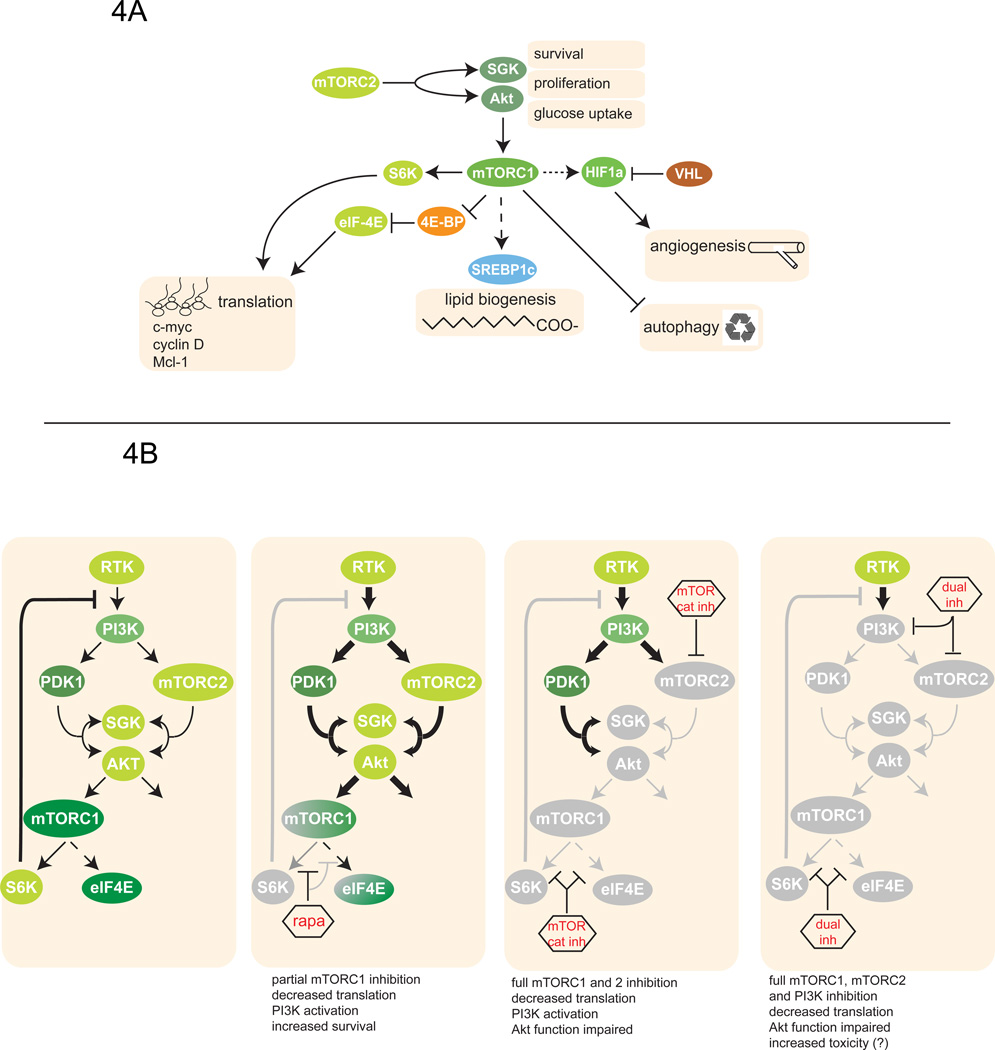
Figure 4. mTOR in cancer.
A. mTOR-regulated cellular processes that play a role in cancer. mTORC1 favors tumorigenesis by driving translation of oncogenes, by inhibiting autophagy, by upregulating angiogenesis, and by enhancing the accumulation of lipids. mTORC2 plays a role in tumorigenesis by activating Akt and other AGC family proteins which promote proliferation and survival. Moreover, by promoting Akt-mediated glucose uptake, mTORC2 fuels the metabolism of cancer cells.
B. Therapeutic inhibition of mTOR activity by rapamycin, mTOR catalytic inhibitors (mTOR cat inh) and dual PI3K-mTOR inhibitors (dual inh). Rapamycin only partially suppresses mTORC1 function, efficiently inhibiting S6K but not eIF4E; thus, it only partially inhibits translation. Moreover, due to the inhibition of the S6K-dependent feedback loops, rapamycin indirectly upregulates phosphatidylinositol 3 kinase (PI3K) activity and promotes cell survival. In contrast, mTOR ATP-competitive inhibitors target all known functions of mTORC1 as well as mTORC2; thus, they inhibit translation more potently. Although PI3K overactivation still occurs, Akt phosphorylation by mTORC2 is impaired. Dual PI3K/mTOR inhibitors block all functions of PI3K, including PDK1- and mTORC2-mediated activation of Akt. However, they might cause increased toxicity.