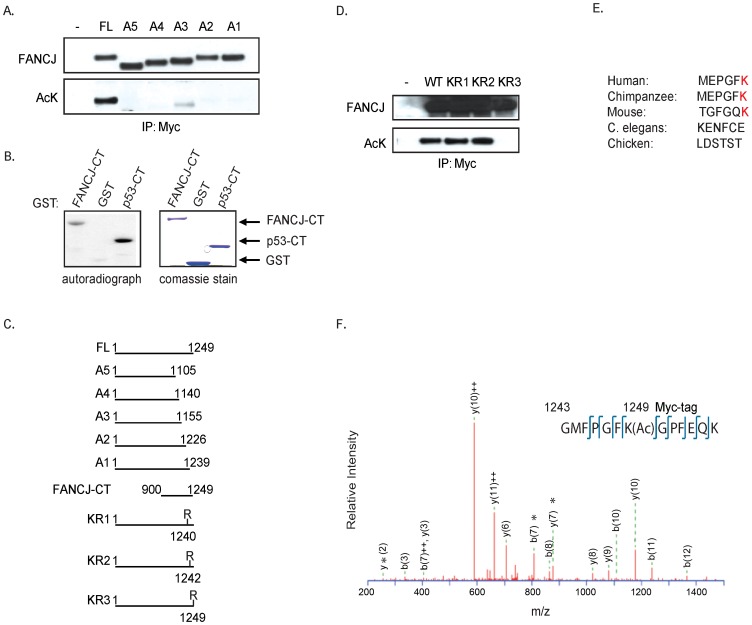
Figure 2. FANCJ is acetylated at lysine 1249.
A. The C-terminus of FANCJ is required for acetylation. Myc-tagged FANCJ mutant constructs were co-expressed with CBP into 293T cells. Cell lysates were collected and analyzed for expression and/or acetylation following immunoprecipitation with the indicated antibodies. B. The FANCJ C-terminus is acetylated in vitro. The recombinant histone acetyltransferase (HAT) domain of p300 was incubated with recombinant FANCJ C-terminal (CT) or p53-CT in the presence of 3H-acetyl CoA. Reaction products were separated by SDS-PAGE and analyzed by autoradiography. Expression of recombinant proteins was determined by Coomassie staining. C. Schematic presentation of wild-type and truncation mutations of FANCJ. D. Lysine 1249 is required for FANCJ acetylation. The Myc-tagged FANCJ mutant constructs noted were co-expressed with CBP into 293T cells and cell lysates were collected and analyzed for expression and/or acetylation following immunoprecipitation with the indicated antibodies. E. Sequence alignment of last 6 residues found in distinct FANCJ species. F. Confirmation of the K1249 acetylation is shown by tandem mass spectrum of FANCJ peptide (amino acid 1243–1249 with Myc tag). *Ions validating localization of acetylation site.