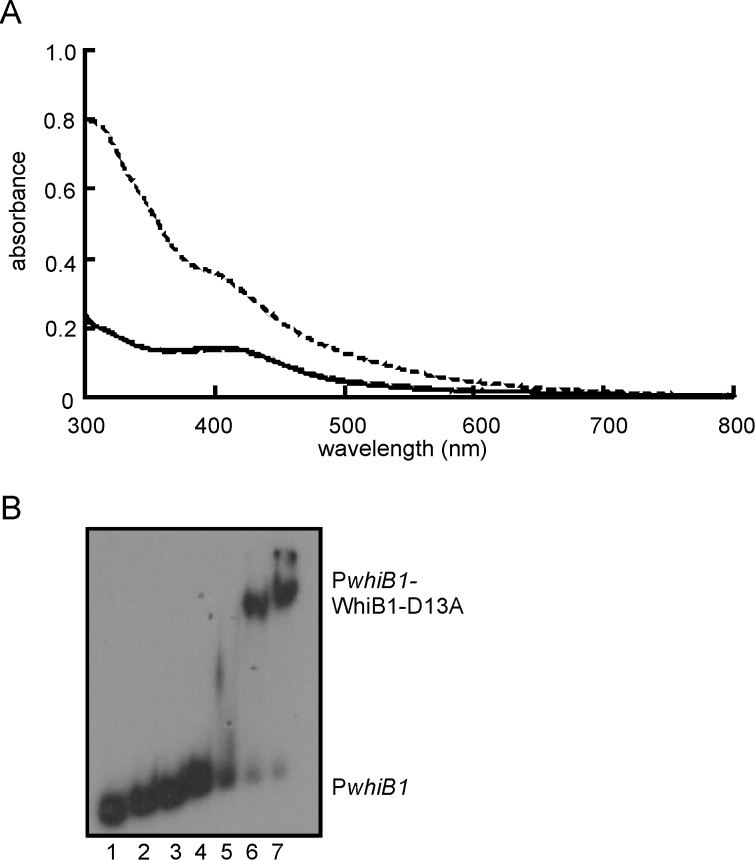
Figure 2. The WhiB1-D13A variant has properties similar to the wild-type protein.
(A) Spectroscopic properties and reactivity of the WhiB1-D13A iron sulfur cluster. The UV-visible spectra of WhiB1-D13A (18.8 μM): after reconstitution (solid line); after 60 min exposure to 110 μM O2 (long-dashed line; mostly superimposed on the solid line); and after 5 min exposure to a 20-fold molar excess of NO (short-dashed line). The buffer was 25 mM Tris-HCl pH 7.4 containing 0.5 M NaCl, 1 mM DTT and 10% glycerol. (B) Apo-WhiB1-D13A binds PwhiB1. Electrophoretic mobility shift assays were as follows: lanes 1, no protein; lanes 2–7, 0.5, 1, 2, 4, 8 and 16 μM WhiB1-D13A, respectively. The locations of PwhiB1 and PwhiB1-WhiB1-D13A complexes are indicated.