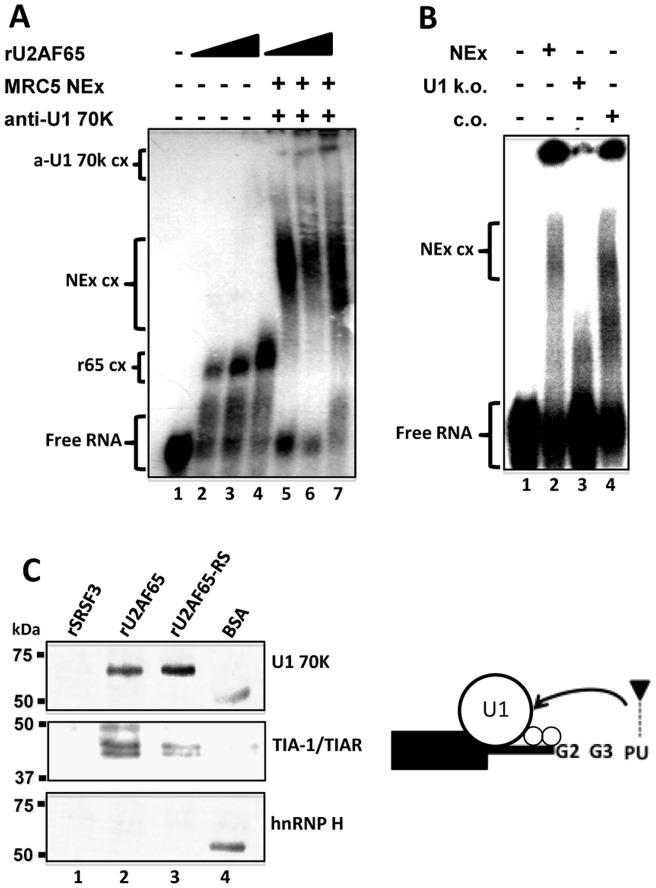
Figure 8. The RS domain of U2AF65 promotes U1 snRNP recruitment to the N50-5'ss.
(A) The G2-G3∼N-PU probe (free probe in lane 1) was used in EMSA experiments with increasing amounts of rU2AF65 (1–3 µg) forming the complexes r65 cx (lanes 2–4, respectively). The same probe was incubated with MRC5 NEx forming the complexes NEx cx (lanes 5–7). NEx cx were supershifted with anti-U1 70K antibodies (a-U1 70k cx) in the presence of 1–3 µg of rU2AF65 (lanes 5–7, respectively). The amount of a-U1 70k cx increased with the amount of rU2AF65. (B) The same probe (free probe, lane 1) was used in EMSA experiments with NEx from H69 cells (lane 2), or with extracts incubated with oligonucleotides targeted to the 5' end of the U1 snRNA and treated with RNase H (U1 k.o.; lane 3), or with RNase H-treated extracts incubated with scrambled oligonucleotides (c.o.; lane 4). Probe and factors depicted as in Fig. 1C. (C) Nickel or protein-A Sepharose (S) beads covered with rSRSF3-(S), rU2AF65, and the SR domain of U2AF65 (rU2AF65-RS) were used in pull-down experiments. U1 70K, TIA-1/TIAR, and hnRNP H (top, middle, and bottom panels, respectively) were monitored by western blots. Both snRNP U1 70K and TIA-1/TIAR were able to interact with U2AF65 and its RS domain (lanes 2, and 3) but not with a different splicing protein, rSRSF3 (lane 1). BSA covered beads were used for blank controls (lane 4); in these lanes protein-A copurified with BSA. No hnRNP H was pulled-down with rU2AF65. The cartoon depicts the possible U1 snRNP and TIA-1/TIAR interactions with U2AF65 in the recognition of a 5'ss.