Figure 1. The molecular structures of PFO and ILY.
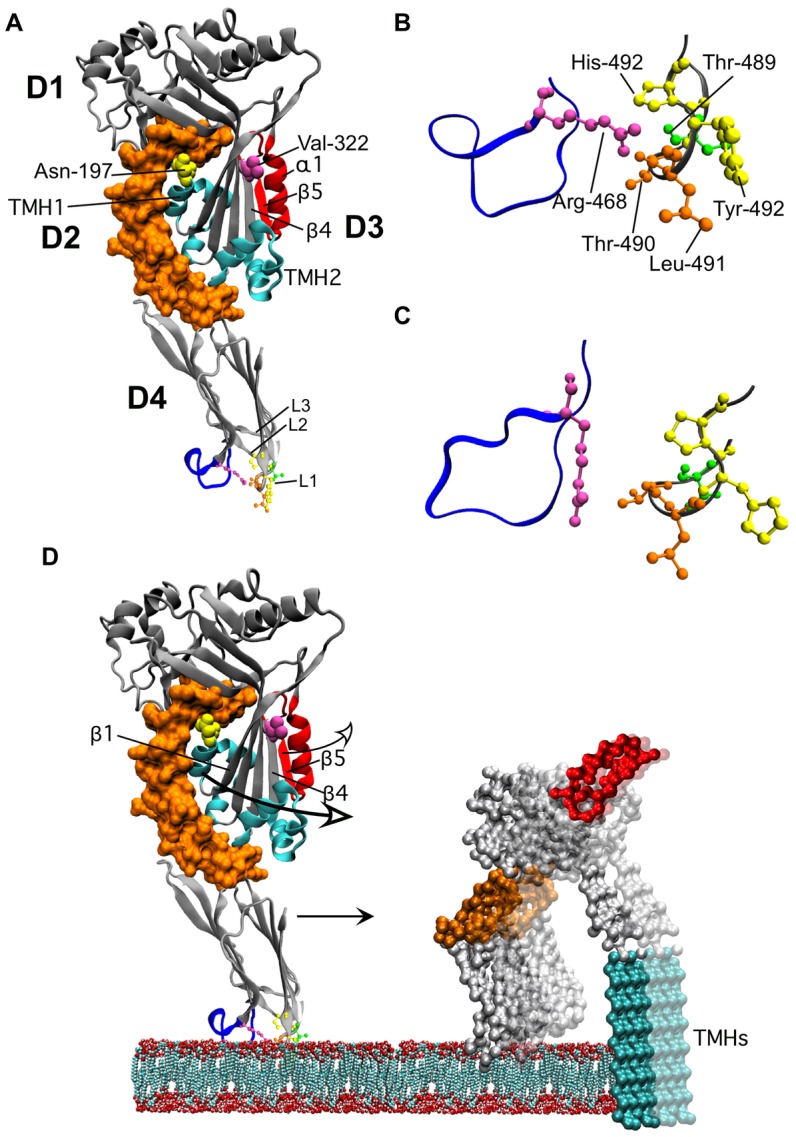
Shown in A is a ribbon representation of the crystal structure of PFO [7]. The domain 3 β5 strand and associated α-helix (α1) that swing away from β4 are highlighted in red. The locations of Asn-197 (at the D2–D3 interface) and Val-322 (buried under the loop formed by α1β5) are shown in space-filled atoms. The twin α-helical bundles in D3 (cyan) extend into the twin transmembrane β-strands (TMHs). The conserved undecapeptide is shown in blue in D4. In panel B an enlarged view of the conserved undecapeptide loop and the CRM containing loop L1 of PFO is shown. In Panel C the analogous structures are shown for ILY as are shown in panel B for PFO.All structures were derived from the crystal structures of PFO and ILY [6], [7]). In panel D we show the structural changes in PFO as it makes the transition from the bound monomer state to the membrane embedded oligomer. The membrane embedded monomer structure is based on the 3D reconstruction of the pneumolysin pore fitted with the PFO crystal structure [57]. D3 breaks its contacts with D2 and swings out in order to extend the α-helical bundles (in cyan) into the twin TMHs. This transition also repositions Asn-197 from the D2–D3 interface to a solvent exposed position within the lumen of the membrane pore. Prior to or simultaneously with the disruption of the D2–D3 interface the α1β5 loop (red) swings away from β4 thus exposing the edge β4 (as well as exposing Val-322 to the solvent), which can then pair with β1 of a second monomer. Upon transition to the pore the oligomeric complex undergoes a 40 Å vertical collapse to insert the β-barrel pore into the bilayer [23]. After β5 breaks contact with β4 the location of α1β5 loop is not known, its position in the model is for illustrative purposes only. All structures were generated using VMD [58].
