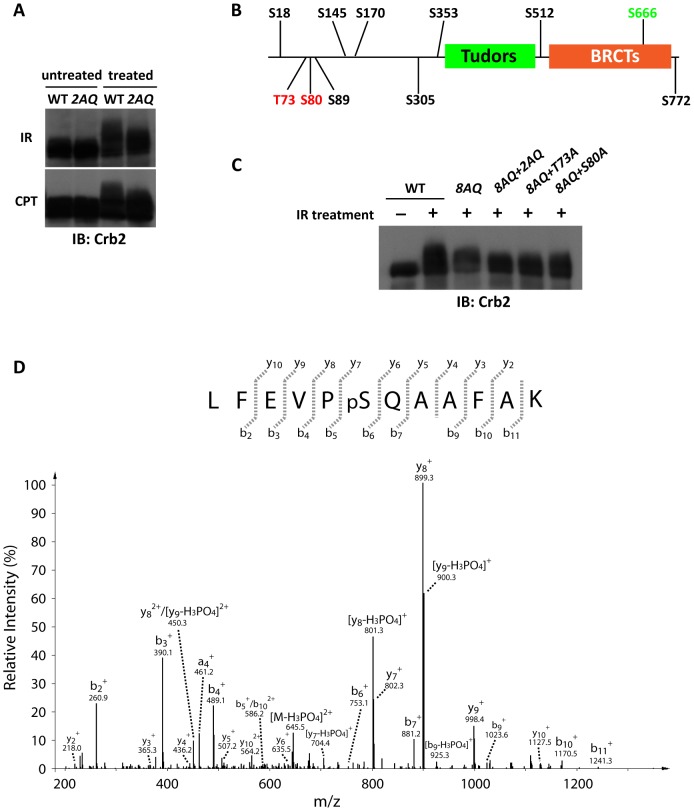
Figure 3. Crb2 SQ/TQ cluster is phosphorylated in vivo.
(A) crb2-2AQ mutations diminished DNA damage-induced Crb2 gel mobility shift. Cells were untreated, or treated with 400 Gy IR or 20 µM CPT. Cell lysates were separated on SDS-PAGE and probed with anti-Crb2 antibody by immunoblotting. Strains used were DY376 and DY383. (B) Locations of eleven SQ/TQ motifs in Crb2. The two conserved residues in the SQ/TQ cluster (T73 and S80) are shown in red, the S666 residue essential for BRCT-mediated dimerization is shown in green, and the other eight SQ/TQs are in black. (C) Mutating the eight SQ/TQs depicted in black in (B) reduced IR-induced Crb2 mobility shift, which could be further attenuated by SQ/TQ cluster mutations. Cells were untreated, or treated with 400 Gy IR. Cell lysates were separated on SDS-PAGE and probed with anti-Crb2 antibody by immunoblotting. Strains used were DY6839, DY6840, DY6843, DY6841 and DY6842. (D) Mass spectrometry analysis showed that S80 residue in Crb2 is phosphorylated. TAP-tagged Crb2 was affinity-purified from cells treated with IR, digested and subjected to mass spectrometry analysis. Phosphorylation of Crb2 at S80 was identified by three overlapping peptides. A representative collision induced dissociation (CID) spectrum of the doubly charged peptide LFEVP[pS]QAAFAK is shown. The strain used was LD412.