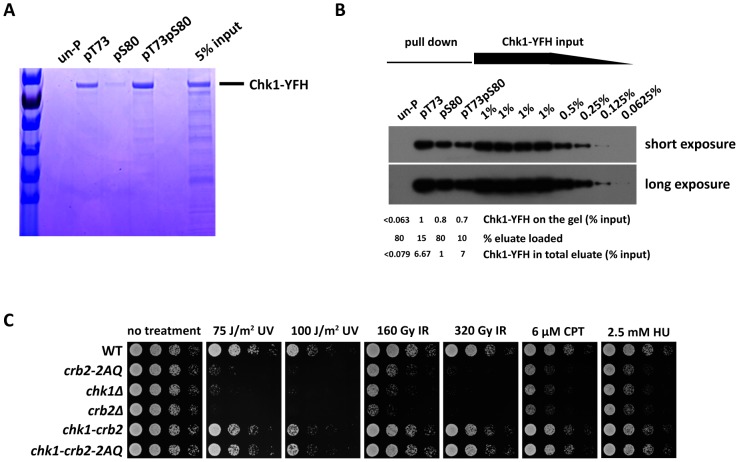
Figure 4. The essential function of the Crb2 SQ/TQ cluster is to mediate a phosphorylation-dependent interaction with Chk1.
(A and B) A 19-amino-acid peptide containing the two conserved SQ/TQ motifs, Crb2(67–85), can bind directly to Chk1 when either T73 or S80 is phosphorylated. Chk1 tagged at its C-terminus with YFP-Flag-His6 (YFH) tag was immunoprecipitated from fission yeast cell extract with anti-Flag beads, eluted with Flag peptide and incubated separately with four types of biotin-labeled Crb2(67–85) peptide, un-phosphorylated (un-P), phosphorylated on T73 alone, S80 alone or both. Peptides were pulled down by streptavidin Dynabeads and eluted by boiling in SDS loading buffer. (A) In one experiment, eluates and input of the peptide pull-down assay were analyzed by 4%–20% SDS-PAGE followed by Coomassie staining. (B) In another experiment, eluates and serial dilutions of Chk1-YFH input were examined by immunoblotting with an anti-GFP antibody. The strain used was DY485. (C) DNA damage sensitivity caused by crb2-2AQ mutation can be fully rescued by fusing Crb2 with Chk1 kinase. Spot assay was performed as in Figure 2B. Strains used were DY6508, DY6509, DY809, DY6507, DY6510 and DY6511.