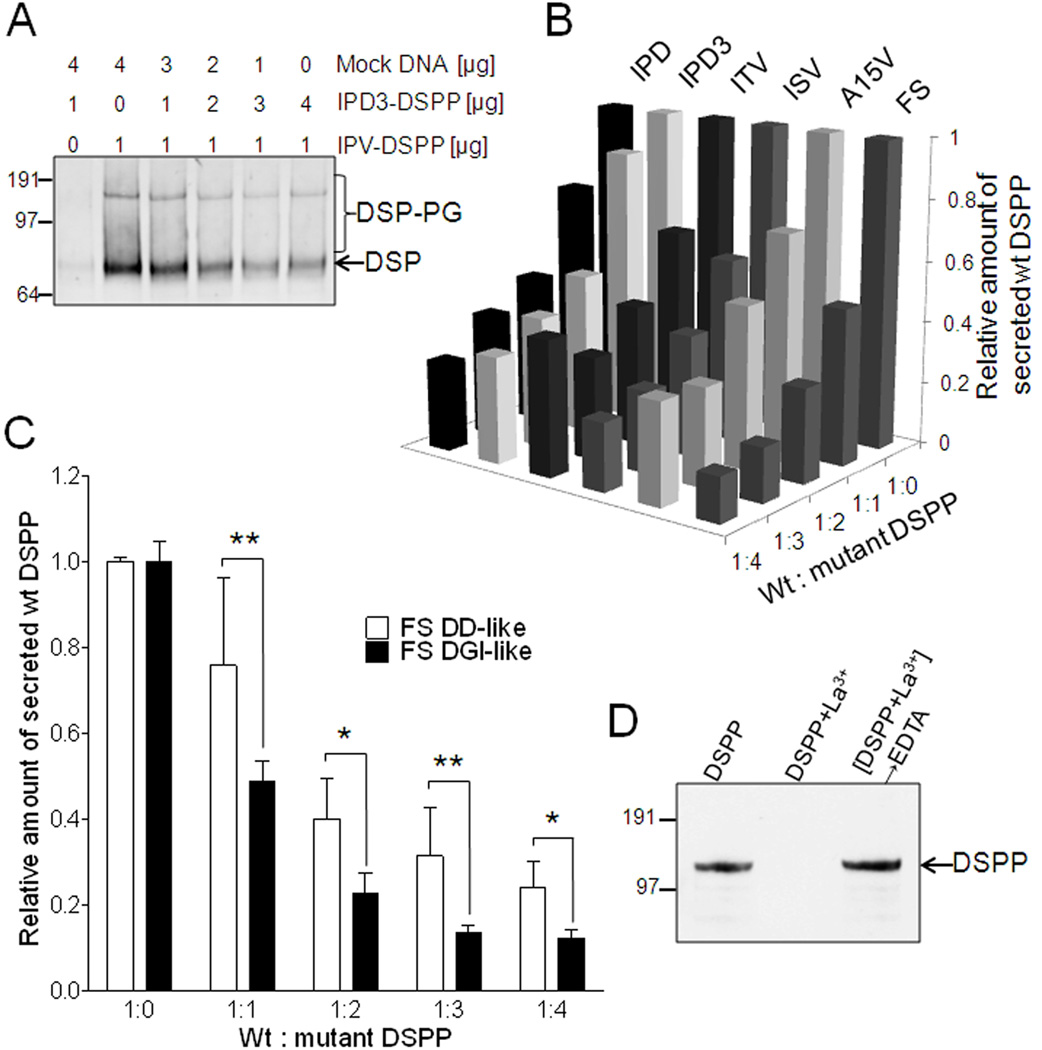
Fig. 6.
Mutant DSPP suppresses wildtype DSPP trafficking/secretion in a dose-dependent manner using multivalent cations. Panel A shows a representative HEK293 cell experiment where increasing doses of mutant DSPP (IPD3) causes the constant amount of expressed WT DSPP to be correspondingly less well secreted into the media as measured by Western blot of total DSP accumulation in media in 48 hr. Co-transfection of FLAG-labeled WT DSPP with increasing doses of six different mutant DSPP (not FLAG-labeled) shows that each of these mutations cause significant decreases in the secretion of WT DSPP into the media as measured by FLAG-anchored sandwich ELISA assay (representative experiment in Panel B). FS mutant DSPP used in panel B had DGI-like number of normal number of SSD repeats amino-terminal to the site of the frameshift. Panel C shows that frameshift mutant DSPP with few normal SSD repeats (FS DD-like) was less effective at reducing the amount of WT DSPP released into the media than a FS with many more normal SSD repeats (FS DGI-like) at each dose of mutation-encoding plasmid in their co-transfection of HEK293 cells. Shown are the FLAG-anchored DPP ELISA results from 24 hr conditioned media (median +/− standard deviation, n=4 experiments with P<0.05 = * and P<0.01 = **). For all experiments above, plasmid containing WT DSPP DNA sequence but lacking eukaryotic promoter (mock DNA) was added to a total 5 µg per transfection with Lipofectamine 2000. FLAG-antibody affinity purified mutant IPD-DSPP (FLAG) by itself remains in the supernatant (detected on Western blot with anti-FLAG antibody, panel D, lane: DSPP) while addition of 10 mM La3+ to duplicate tube caused complete loss of DSPP in supernatant by 100,000 × g centrifugation for 2 hr (lane: DSPP+La3+). The IPD-DSPP could be recovered from the La3+-induced pellet by subsequent addition of excess EDTA to the La3+ tube (lane: DSPP+La3+ → EDTA).