Fig. 7.
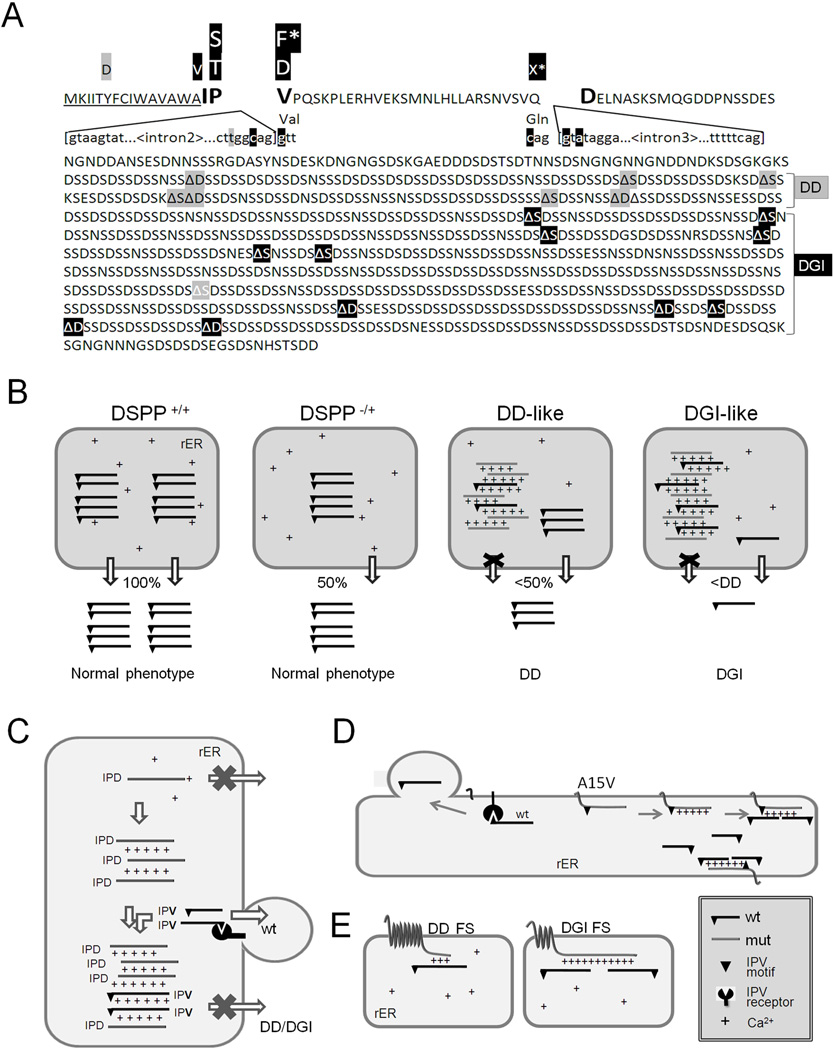
DSPP mutations that cause nonsyndromic DD/DGI and models illustrating why they are dominant negative. Panel A shows the locations of DSPP missense/nonsense mutations above the wildtype protein sequence. The leader peptide is underlined. IPV motif (plus D beginning exon 4 that result in IPD3 motif when exon 3 is skipped) are shown in a larger font. Splice site-relevant sequences from introns 2 and 3 (plus bases for first and last codons of exon 3) are shown in lower case. Grey highlights denote DD-associated mutations (e.g., 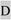 and
and  for amino acid changes and
for amino acid changes and  for base changes leading to skipping of exon 3) and dark highlights (e.g.,
for base changes leading to skipping of exon 3) and dark highlights (e.g., 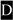 ,
, 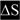 , and with
, and with  ,)respectively) for DGI-associated mutations. The ΔS and ΔD represent locations of frameshift mutations. Letters with asterisk (F* and X*) are missense and nonsense codons respectively, that result in skipping of exon 3. Note that the frameshift mutations causing DD cluster in the 5′ region of repeat domain while DGI are more 3′. References for mutations are cited in the text. The rER of WT odontoblasts produce and traffic the full complement of normal DSPP protein (+/+ in Panel B). Complete loss of one allele (DSPP −/+) results in only half the amount of normal DSPP exiting the rER, but the phenotype remain normal. Expression of a DD-like mutant protein from one allele causes some WT DSPP to also be retained within the rER (DSPP, DD-like) thereby causing the mild DD. In contrast, other mutant alleles produce a DSPP protein that poorly exits the rER and more effectively captures the WT DSPP (DSPP, DGI-like) causing the more severe DGI (B). Unblocked arrow (⇩) denotes successful trafficking of WT DSPP (IPV) out of the rER. Changes in the “IPV” motif (▼) results in a mutant proteins (grey and lacking the ▼ in panel C) which cannot interact with its proposed receptor and form calcium (+) bridged complexes that then adsorb WT DSPP, thereby also keeping the normal protein from trafficking out of the rER. Retention of the hydrophobic leader sequence by A15V causes the mutant protein to become embedded within the rER membrane as a single-pass transmembrane protein where it entraps WT DSPP through the same Ca2+ bridges (D). Similarly, the mutant hydrophobic domains of both DD-FS and DGI-FS DSPP become embedded in rER membrane (E). DD-FS, having shorter normal Ca2+-binding domains remaining, are less efficient at entrapping WT DSPP than the DGI-FS with its much longer negatively charged SSD repeat domain (E).
,)respectively) for DGI-associated mutations. The ΔS and ΔD represent locations of frameshift mutations. Letters with asterisk (F* and X*) are missense and nonsense codons respectively, that result in skipping of exon 3. Note that the frameshift mutations causing DD cluster in the 5′ region of repeat domain while DGI are more 3′. References for mutations are cited in the text. The rER of WT odontoblasts produce and traffic the full complement of normal DSPP protein (+/+ in Panel B). Complete loss of one allele (DSPP −/+) results in only half the amount of normal DSPP exiting the rER, but the phenotype remain normal. Expression of a DD-like mutant protein from one allele causes some WT DSPP to also be retained within the rER (DSPP, DD-like) thereby causing the mild DD. In contrast, other mutant alleles produce a DSPP protein that poorly exits the rER and more effectively captures the WT DSPP (DSPP, DGI-like) causing the more severe DGI (B). Unblocked arrow (⇩) denotes successful trafficking of WT DSPP (IPV) out of the rER. Changes in the “IPV” motif (▼) results in a mutant proteins (grey and lacking the ▼ in panel C) which cannot interact with its proposed receptor and form calcium (+) bridged complexes that then adsorb WT DSPP, thereby also keeping the normal protein from trafficking out of the rER. Retention of the hydrophobic leader sequence by A15V causes the mutant protein to become embedded within the rER membrane as a single-pass transmembrane protein where it entraps WT DSPP through the same Ca2+ bridges (D). Similarly, the mutant hydrophobic domains of both DD-FS and DGI-FS DSPP become embedded in rER membrane (E). DD-FS, having shorter normal Ca2+-binding domains remaining, are less efficient at entrapping WT DSPP than the DGI-FS with its much longer negatively charged SSD repeat domain (E).