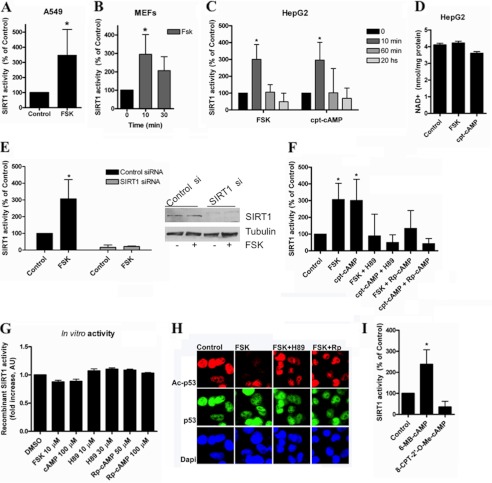
FIGURE 2.
cAMP/PKA increase SIRT1 activity by mechanism that is independent of changes in NAD levels. SIRT1 activity was measured in A549 cells (A), mouse embryonic fibroblasts (B), and HepG2 cells (C) treated with 10 μm forskolin (FSK) or 100 μm cpt-cAMP for the indicated times. *, p < 0.01 (ANOVA, n = 3–9). SIRT1 activity was normalized to time 0. D, -fold change in NAD+ concentration in HepG2 cells after forskolin (10 μm) or cpt-cAMP (100 μm) incubation for 10 min (n = 3). E, SIRT1 was knocked down in HepG2 cells with specific siRNA, and SIRT1 activity was assessed after stimulation with 10 μm forskolin for 10 min. Activity is shown as -fold change with respect to the control. *, p < 0.05 (ANOVA test, n = 3). Cell lysates were immunoblotted with anti-SIRT1 and anti-tubulin antibodies. F, cells were pretreated for 45 min with the PKA inhibitor H89 (30 μm) or (Rp)-cAMP (100 μm) and then stimulated with 10 μm forskolin or 100 μm cpt-cAMP for 10 min. SIRT1 activity was normalized to the control. * shows significant difference with respect to the control (p < 0.01, ANOVA, n = 3). G, human recombinant purified SIRT1 activity in the presence of different compounds was measured in vitro. 0.2 unit of SIRT1 was incubated with the compounds at the indicated concentrations. H, HepG2 cells were pretreated for 45 min with the PKA inhibitor H89 (30 μm) or (Rp)-cAMP (Rp; 100 μm) and then stimulated with 10 μm forskolin for 10 min. Immunofluorescence for Ac-p53 and total p53 was analyzed using specific antibodies. I, cells were incubated with the PKA activator 6-MB-cAMP (100 μm) and the EPAC activator 8-CPT-2′-O-Me-cAMP; (100 μm) for 10 min before harvesting. SIRT1 activity was measured and normalized to the control. *, p < 0.01 (t test, n = 3). Error bars represent S.D. AU, arbitrary units.