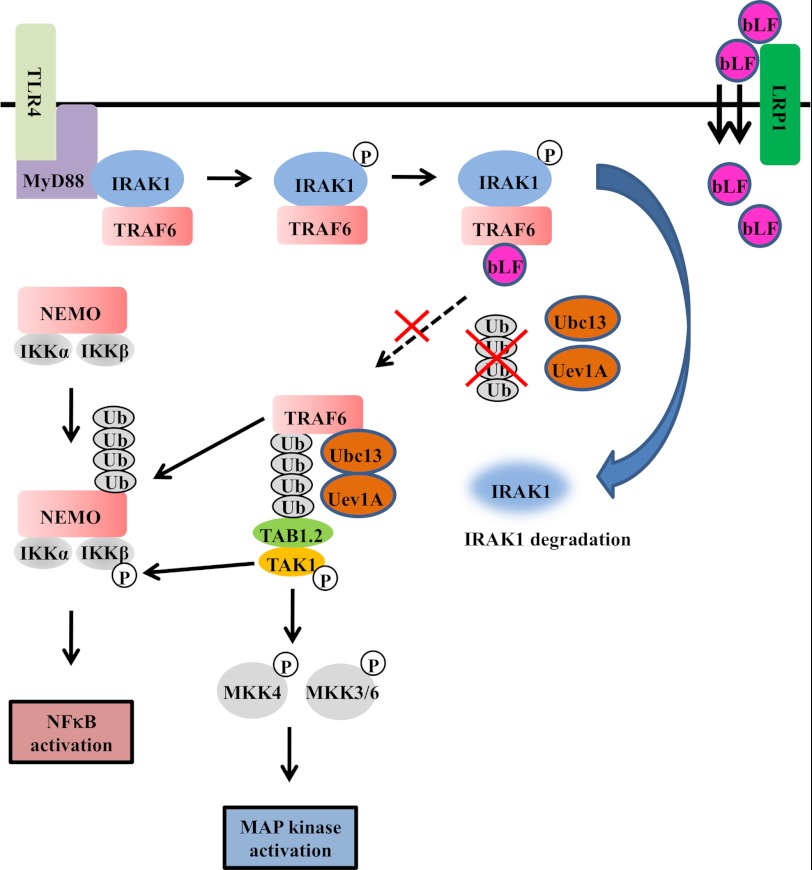
FIGURE 8.
The molecular mechanisms behind the effects of bLF on LPS-mediated osteoclastogenesis. The binding of LPS to TLR4 initiates the formation of a complex that includes MyD88 and IRAK1. IRAK1 undergoes autophosphorylation shortly after LPS stimulation. Hyperphosphorylated IRAK1 then disengages from MyD88 and forms a cytosolic IRAK1-TRAF6 complex. TRAF6 is then autoubiquitinated and interacts with TAK and TAB. Phosphorylated IRAK1 also undergoes ubiquitin-mediated degradation. Once TAK1 is phosphorylated subsequent to TRAF6 activation, it activates the NFκB pathway by phosphorylating IKKβ, for which TRAF6-mediated polyubiquitination of NEMO (NFκB essential modulator) is necessary. Active TAK1 also phosphorylates the MAPK kinases MKK4 and MKK3/6, which activate p38 and JNK, respectively. Endocytosed bLF through LRP1 binds to TRAF6 after bLF pretreatment. In bLF-pretreated cells, LPS stimulation induces IRAK1 degradation subsequent to its kinase activation. In contrast, bLF inhibits TRAF6 autoubiquitination. NEMO, NFκB essential modulator.